|
Manufacturing Process
|
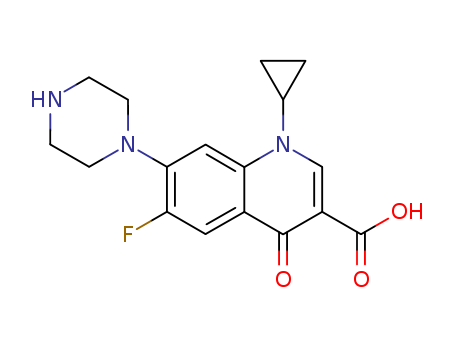
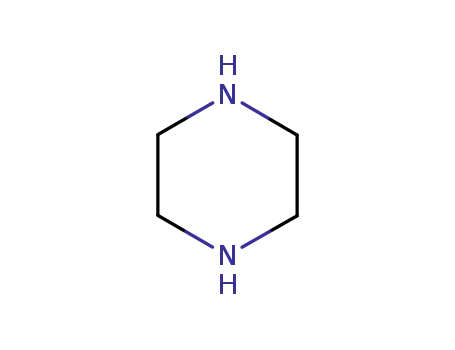
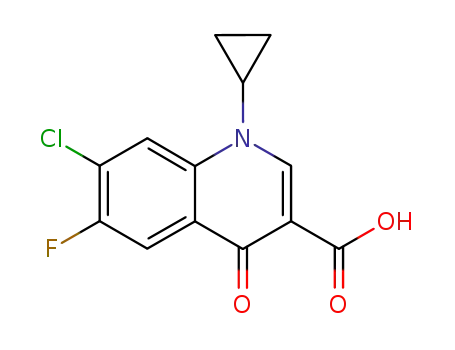
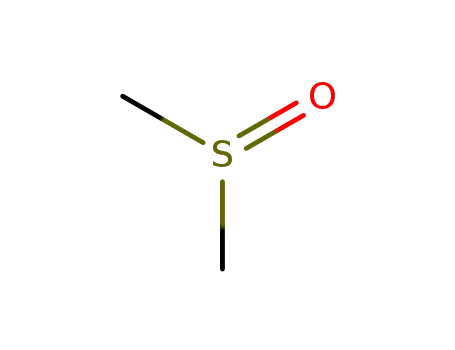
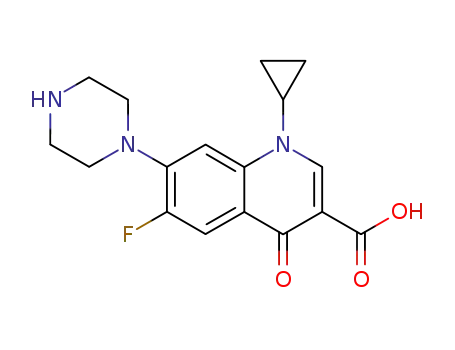
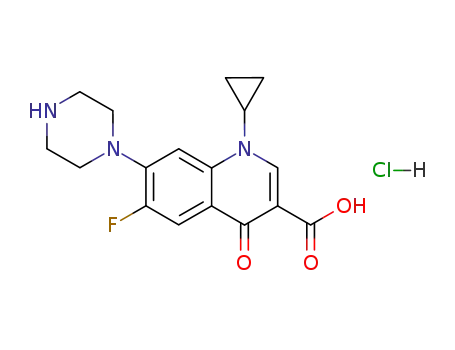
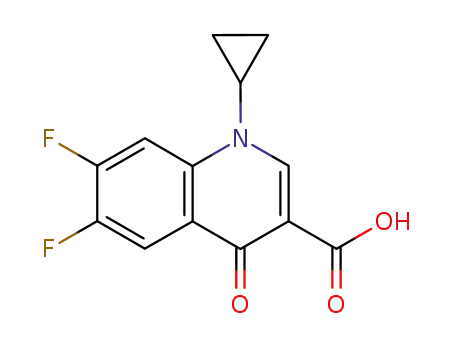
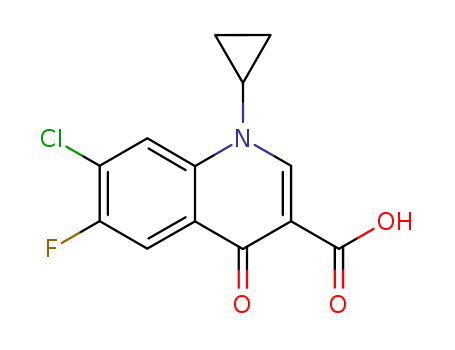
Cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-quinolinecarboxylic
acid was synthesized by heating of a mixture of 7-chloro-1-cyclopropyl-6-
fluoro-1,4-dihydro-4-oxo-quinolin-3-carboxylic acid and 30.1 g dry piperazine
in 100 ml DMSO for 2 hours at 135-140°C. DMSO was evaporated in high
vacuum. The residue was heated with 100 ml of water, and was dried over
CaCl2 in vacuum. Cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-
quinolinecarboxylic acid obtained has a temperature of decomposition 255-
257°C. |
|
Therapeutic Function
|
Antibiotic |
|
Antimicrobial activity
|
It exhibits potent activity against most Enterobacteriaceae, as well as against Acinetobacter spp. (MIC 0.25–1 mg/L), fastidious Gram-negative bacilli such as Mor. catarrhalis (MIC 0.06–0.25 mg/L) and Campylobacter jejuni(MIC 0.12 mg/L). In common with other quinolones, it is active against Bacillus anthracis. Ciprofloxacin is the most active quinolone against Ps. aeruginosa and exhibits good activity in vitro against other non-fermenting Gram-negative bacilli. In-vitro activity against Staph. aureus coagulase-negative staphylococci, Str. pyogenes, Str. pneumoniae and Enterococcus spp. (MIC c. 0.5–2 mg/L) is moderate. Most methicillin-resistant strains of staphylococci are resistant. It has poor activity against anaerobes, but is active against M. tuberculosis, Mycoplasma spp. and intracellular pathogens such as Chlamydia, Chlamydophila and Legionella. |
|
Pharmaceutical Applications
|
A 6-fluoro, 7-piperazinyl quinolone formulated as the hydrochloride for oral administration and as the lactate for intravenous use. |
|
Pharmacokinetics
|
Oral absorption: 50–80% Cmax 500 mg oral: 1.5–2 mg/L after 1–2 h 200 mg intravenous (15-min infusion): 3.5 mg/L end infusion Plasma half-life: 3–4 h Volume ofdistribution: 3–4 L/kg Plasma protein binding: 20–40%AbsorptionAfter escalating oral doses, mean peak plasma levels increase proportionately with dose. However, accumulation occurs after repeated doses of 500 mg orally or 200 mg intravenously every 12 h: the apparent elimination half-life has been reported to rise to about 6 h after a regimen of 250 mg every 12 h for 6 days. Absorption is delayed, but seems unaffected by food and, in common with other quinolones, is reduced by certain antacids. Co-administration of sucralfate reduces the peak plasma concentrations to undetectable levels in many subjects (mean value from 2 to 0.2 mg/L) and the AUC is reduced to 12% of the value obtained when ciprofloxacin is administered alone. Ferrous sulfate and multivitamin preparations containing zinc significantly reduce absorption, which is also impaired in patients receiving cytotoxic chemotherapy for hematological malignancies. Calculated total bioavailability is 60–70%.DistributionIt is widely distributed in body fluids, concentrations in most tissues and in phagocytic cells approximating those in plasma. Concentrations in the CSF, even in the presence of meningitis, are about half the simultaneous plasma levels.Metabolism and excretion It is partly metabolized to four metabolites, all but one of which (desethylciprofloxacin) display antibacterial activity. About 95% of a dose can be recovered from feces and urine. Around 40% of an oral and 75% of an intravenous dose appear in the urine over 24 h. Elimination is by both glomerular filtration and tubular secretion (60–70%) and is reduced by concurrently administered probenecid and by renal insufficiency. It is poorly removed by hemodialysis. Part of the administered drug is eliminated in the bile. An enterohepatic cycle results in prolongation of the half-life. The four metabolites are eliminated in the urine and feces at low concentration in comparison to the parent compound. |
|
Side effects
|
Untoward reactions are uncommon, those encountered being typical of the group. Reactions severe enough to require withdrawal of treatment have occurred in <2% of patients. The most common reactions, gastrointestinal tract disturbances, have been seen in 5% of patients and rashes in about 1%. CNS disturbances typical of quinolones have been reported in 1–2% of patients. Tendinitis and tendon rupture (especially of the Achilles tendon) may occur in a small number of patients and ciprofloxacin should be avoided in patients at risk for these conditions. Potentiation of the action of theophylline and other drugs metabolized by microsomal enzymes may occur. Crystalluria and transient arthralgia have been reported.In volunteers, dosages of up to 750 mg produced no change in the numbers of fecal streptococci and anaerobes, but did produce a 2.5 × log10 decline in the numbers of enterobacteria, which lasted 1 week. There was no change in the susceptibility of the affected organisms and no overgrowth by resistant strains. As with other quinolones, ciprofloxacin is not recommended for use in children or in pregnant or lactating women.The drug should be avoided in suspected or confirmed infections caused by Str. pneumoniae. It is inferior to conventional agents and some other fluoroquinolones in the treatment of genital tract infections caused by C. trachomatis.Ciprofloxacin has also been shown to be effective in the treatment of patients with malignant otitis externa, catscratch disease, prevention of infection in patients undergoing biliary tract surgery, and treatment of biliary tract infections. A topical preparation for use in the treatment of ocular infections is available, but is neither more effective nor safer than established topical agents; it may be indicated for superficial eye infections caused by pathogens resistant to conventional drugs or in patients unable to tolerate standard therapeutic agents. |
|
Synthesis
|
Ciprofloxacin, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piper�azinyl)-3-quinolincarboxylic acid (33.2.19), is synthesized in a completely analogous
scheme, except that instead of using ethyl iodide in the alkylation stage, cyclopropyl
bromide is used. |
|
Veterinary Drugs and Treatments
|
Because of its similar spectrum of activity, ciprofloxacin could be
used as an alternative
to enrofloxacin when a larger oral dosage
form or intravenous product is desired. But the two compounds
cannot be considered equivalent because of pharmacokinetic differences
(see below). |
|
Drug interactions
|
Potentially hazardous interactions with other drugs
Aminophylline and theophylline: possibly increased
risk of convulsions; increased levels of aminophylline
and theophylline.
Analgesics: increased risk of convulsions with
NSAIDs.
Anticoagulants: anticoagulant effect of coumarins
enhanced.
Antidepressants: metabolism of duloxetine inhibited
- avoid; avoid with agomelatine.
Antimalarials: manufacturer of artemether with
lumefantrine advises avoid concomitant use.
Antipsychotics: possibly increased concentration of
olanzapine and clozapine.
Ciclosporin: variable response; no interaction seen
locally; some reports of increased nephrotoxicity.
Clopidogrel: possibly reduced antiplatelet effect.
Cytotoxics: possibly increased concentration of
bosutinib, ibrutinib and olaparib - avoid or consider
reducing dose of bosutinib; possibly reduced
excretion of methotrexate; concentration of erlotinib
increased.
Muscle relaxants: tizanidine concentration increased
- avoid.
Pirfenidone: concentration of pirfenidone increased
- reduce dose of pirfenidone.
Tacrolimus: increased levels (anecdotally). |
|
Environmental Fate
|
The antimicrobial action of the drug is due to inhibition of the
enzymes required for bacterial DNA function. Topoisomerase
II (DNA gyrase) and topoisomerase IV are necessary for
bacterial DNA replication, transcription, strand repair, and
recombination. Thus, ciprofloxacin cytotoxicity may be caused
by the loss of mtDNA encoded functions. |
|
Metabolism
|
Ciprofloxacin is eliminated principally by urinary
excretion, but non-renal clearance may account for about
one-third of elimination and includes hepatic metabolism,
biliary excretion, and possibly transluminal secretion
across the intestinal mucosa. At least 4 active metabolites
have been identified. Oxociprofloxacin appears to be
the major urinary metabolite and sulfociprofloxacin the
primary faecal metabolite.Urinary excretion is by active tubular secretion as well
as glomerular filtration and is reduced by probenecid;
it is virtually complete within 24 hours. About 40-50%
of an oral dose is excreted unchanged in the urine and
about 15% as metabolites. Up to 70% of a parenteral dose
may be excreted unchanged within 24 hours and 10% as
metabolites. Faecal excretion over 5 days has accounted
for 20-35% of an oral dose and 15% of an intravenous
dose. |
|
Definition
|
ChEBI: A quinolone that is quinolin-4(1H)-one bearing cyclopropyl, carboxylic acid, fluoro and piperazin-1-yl substituents at positions 1, 3, 6 and 7, respectively. |
|
Brand name
|
Cipro (Bayer);CIPROBAY. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego