|
Antispasmodic drugs
|
Baclofen is a γ-aminobutyric acid derivative. It is an antispasmodics class drugs acting on the central nervous system, the brain and spinal cord as a skeletal muscle relaxants and sedatives. It take antispasmodic effect by stimulating receptors to inhibit the release of excitatory amino acids such as glutamic acid, aspartic acid and thereby inhibiting the transfer of single and multi-synaptic reflex in central nervous system, brain and spinal cord. Clinically it has already been used as racemic agent for treating Muscle spasticity since mid-1960s. Recent studies have also found that this product can significantly reduce the symptoms of gastroesophageal reflux and alleviating related symptoms. It can also alleviate the dystonia of children and treat the urination dysfunction caused by central spinal cord injury. In addition, the intrathecal injection therapy of the product in clinical application can further improve the clinical efficacy, and make it possible to adjust drugs dosage which stabilizes the treatment effect. For the past 10 years, the clinical application of central muscle relaxant baclofen has made significant progress, particularly in accumulating a lot of experiences in the neurological rehabilitation treatment of reducing muscular tension and pain alleviation treatment.
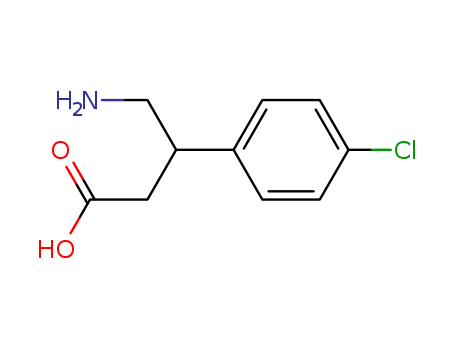
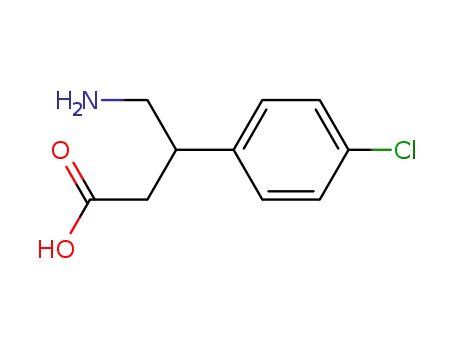
Figure 1 Structure of Baclofen |
|
Mechanisms of action
|
The content of γ-aminobutyric acid (GABA) is very high in the human central nervous system, brain and spinal cord. Its level is 1000 times higher than monoamine such as catecholamines, to higher norepinephrine, and dopamine. It is presented at the highest inside the substantia nigra and globus pallidus. There are about 20% to 40% of the synapses using GABA as neurotransmitter inside the brain. GABA is the major inhibitory neurotransmitter of the central nervous system, brain and spinal cord. However, it is not able to penetrate the blood-brain barrier. GABA can be converted into baclofen by connecting carbon atom to the p-chlorophenyl. In this case, it changes from hydrophilic substance to lipophilic substances, which can then go through the blood-brain barrier. The main effect of this product are stimulating the GABA receptors, inhibiting the release of excitatory amino acids such as aspartic acid, glutamic acid, also cause the efflux of K +, Ca 2+ , which results in hyper-polarization, reducing the transfer single and multi-synaptic reflex, maintaining the normal function of middle-neuronal activity in order to reduce the activity of motor neurons, thereby alleviating skeletal muscle spasticity caused by damage to the pyramidal tract, reducing muscle tension, and promoting sports functional recovery.
The above information is edited by the lookchem of Dai Xiongfeng. |
|
Production process
|
Chlorobenzaldehyde and ethyl acetoacetate are condensed into chlorobenzene methylene-bis-ethyl acetoacetate. Heat and hydrolyze to obtain chlorophenyl glutaric acid. Use acetic anhydride for dehydration and cyclization to obtain chlorophenyl glutaric anhydride. And then perform amination reaction by concentrated aqueous ammonia to generate chlorophenyl glutaric acid imide. The open the ring, degrade to obtain the final product. |
|
Pharmacokinetics
|
After oral administration, concentration in plasma reaches a peak in about 1.9h. There is large fluctuation in plasma concentration with the maximum concentration/minimum concentration × 100%: 188-439%. The total clearance rate is about 175mL.min-1, and the apparent renal clearance crisp is the same as that of muscle. The plasma protein binding rate is 35%. The excretion rate of kidney prototype drug is 65%, which is consistent with healthy results. Because renal excretion is the major route of elimination, patients with renal impairment should pay attention to adjust the dose. |
|
Indications
|
This product is an antispasmodic drug. It inhibits the transmission in spinal cord of mono-synapse multiple synapse. The mechanism is inhibiting the release of excitatory amino acid glutamate and aspartate through stimulating GABA receptor. Thus reduce the increased limbs muscle tension caused by spinal cord lesions, multiple sclerosis, and spinal cord injury. It can be used for treatment of muscle spasms caused by brain and spinal cord diseases or injuries. |
|
Precautions
|
1. Among the overdose behavior, the depression of central nervous system is the most prominent. Severe poisoning manifestations include seizures, coma, respiratory depression and muscle hypotonia associated with loss of reflexes of the limbs, and also hypotension and low blood pressure sometimes. Strategies for emergence treatment of acute poisoning include respiratory support, gastric lavage, and diuretic. However, there is no special antidote reported. There are some reports that seizures and myoclonic seizures appeared in some patients appear during the recovery period. Epileptic seizure can be treated by diazepam or clonazepam, although these drugs can cause extension of the duration of unconsciousness. Patients of cardiovascular disease should be carefully observed for 1 week to monitor delayed tachycardia and hypertension.
2. Patients who are allergic to the drug, suffering Parkinson's disease, spasms, and the women who are in the first three months of pregnancy are not allowed for using.
3. Patients of hypertension, peptic ulcer disease, cerebrovascular disease and respiratory, issue liver and kidney dysfunction, who have a history of seizures or convulsions, accompanied by mental disorders, schizophrenia or confusion, pregnant and lactating women, drivers or operator of machine should use with caution.
4. Epileptic patients should be controlled of the attack of epilepsy when applying this drug. Also they should do EEG monitoring.
5. Gradually reduce the dosage before the withdrawal, to prevent rebound phenomenon. |
|
Side effects
|
The main side effects mainly happen at the beginning of treatment when the dose increases too fast, when overdose happens and for elderly patients. It is mainly mild transient symptoms, Patients with historical mental illness and elderly patients with cerebral vascular disease may suffer from an even more severe adverse reaction. During the beginning of treatment, there are often some side effects such as daytime sedation, drowsiness and nausea. |
|
Dosage and usage
|
Oral: adult, Initial amount of 5mg per time, tid, then every increase taking this amount every 3d until it reaches the appropriate dose 30~75mg/d. Take this in 3 times. The dose should not exceed 80 mg/d except in special cases. Gradually reduce the dosage before withdrawal; For children, the initial amount should be 0.3mg/(kg.d), and the maintenance dose should be 0.75~2mg/(kg.d). For children under 10 years-old, the maximum dose should be 2.5mg /(kg.d). The recommended daily amount for maintenance therapy: 1 to 2 years-old, 10~20mg, 2~10 years-old: 30~60mg (maximum 70mg), take it with meal or with milk. |
|
Production method
|
Chlorobenzaldehyde and ethyl acetoacetate is condensed into chlorobenzene methylene-bis-ethyl acetoacetate. Add heating water for hydrolysis to obtain chlorophenyl glutaric acid. Dehydrate and cyclize to obtain chlorophenyl glutaric anhydride using acetic anhydride. Then perform amination reaction with concentrated aqueous ammonia to generate chlorophenyl glutaric acid imide. The open the ring, degrade to obtain the final product. Detailed procedures as follow: Add 42.25g β-(p-chlorophenyl) glutaric acid imide with stir to 200 mL aqueous solution containing 8.32g of sodium hydroxide. The mixture was heated at 50 °C for 10min, cool to 10-15 °C. Titrate for 200 mL aqueous solution of 40.9g of sodium hydroxide. After 20min, add 38.8g bromine, stir at 20-25 °C for 8h. Use concentrated hydrochloric acid to adjust the pH of the reaction solution to 7, and then precipitate out the fine crystals of i.e. [beta] (p-chlorophenyl) amino-gamma-acid. Re-crystallize in water with m.p. 206-208 °C. |
|
Toxicity grading
|
highly toxic |
|
Acute toxicity
|
Oral-rat LD50; 145 mg/kg; Oral-Mouse LD50: 200 mg/kg. |
|
Flammability and hazardous characteristics
|
Combustible, Burning yields toxic chloride fumes and nitrogen oxides; Patients takes with side effects: low blood pressure, pulse rate decreases, muscle weakness. |
|
Storage Characteristics
|
ventilation, low-temperature and dry; and Separate from food raw materials for storage. |
|
Extinguishing agent
|
Dry powder, foam, sand, carbon dioxide, water spray. |
|
Manufacturing Process
|
42.45 g of β-(p-chlorophenyl)glutaric acid imide are stirred into a solution of
8.32 g of sodium hydroxide in 200 ml of water. The mixture is heated for 10
minutes at 50°C, and the solution thus formed is cooled to 10° to 15°C. At
this temperature there are then added dropwise a solution of 40.9 g of
sodium hydroxide in 200 ml of water and then, in the course of 20 minutes,
38.8 g of bromine. When all has been dropped in, the batch is stirred for 8
hours at 20° to 25°C. The reaction solution is then cautiously adjusted with
concentrated hydrochloric acid to pH 7, whereupon finely crystalline γ-amino-
β-(p-chlorophenyl)butyric acid settles out. To purify it, it is recrystallized from
water. Melting point is 206°to 208°C. |
|
Therapeutic Function
|
Muscle relaxant |
|
Biological Functions
|
Baclofen (Lioresal) is the parachlorophenol analogue
of the naturally occurring neurotransmitter γ-aminobutyric
acid (GABA). |
|
Air & Water Reactions
|
Insoluble in water. |
|
Reactivity Profile
|
Baclofen is an amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
|
Health Hazard
|
SYMPTOMS: Symptoms of exposure to Baclofen via ingestion may include drowsiness, insomnia, dizziness, weakness, mental confusion, nausea, constipation, anorexia, urinary retention, impotence, nystagmus, diplopia and incoordination. Ingestion may lead to cholinergic effects and lassitude. It may also lead to ataxia. Other symptoms due to ingestion may include impaired renal function, fatigue, headache, hypotension, urinary frequency, rash, pruritis, ankle edema, excessive perspiration, weight gain, nasal congestion, and rarely, euphoria, excitement, depression, hallucinations, paresthesia, muscle pain, tinnitus, slurred speech, tremor, rigidity, dystonia, blurred vision, strabismus, miosis, mydriasis, dysarthia, epileptic seizure, dyspnea, palpitation, chest pain, syncope, dryness of the mouth, taste disorder, abdominal pain, vomiting, diarrhea, blood in the stools, enuresis, dysuria, inability to ejaculate, nocturia and hematuria. Overexposure through ingestion may result in seizures, and coma with respiratory depression. Aspiration pneumonia is a frequent complication of coma with respiratory depression. Other symptoms due to overdosage may include vomiting, muscular hypotonia, drowsiness, and accommodation disorders. Cyanosis has been reported. Chronic ingestion may result in drowsiness, depression, weakness, anxiety, ataxia, headaches, blurred vision, gastric upset and pruritic skin rashes characterized by urticaria or erythematous macular eruptions. Sudden withdrawal after chronic ingestion may cause auditory and visual hallucinations, anxiety and tachycardia. Seizures may also occur after sudden withdrawal. Abuse may lead to drug dependence. |
|
Fire Hazard
|
Flash point data for Baclofen are not available. Baclofen is probably combustible. |
|
Biological Activity
|
Selective GABA B receptor agonist. Skeletal muscle relaxant. |
|
Biochem/physiol Actions
|
Baclofen, a γ-aminobutyric acid (GABA) analog, possesses myorelaxant properties Being a γ-aminobutyric acid receptor B (GABAB) agonist, baclofen may be involved in the potentiation of dendritic potassium K+?channels. It may be useful in blocking transient lower esophageal sphincter relaxation (TLESR)?in gastroesophageal reflux disease (GERD). Baclofen may also have scope for treating addictive especially, in alcohol use disorder (AUD). |
|
Mechanism of action
|
Baclofen appears to affect the neuromuscular axis by
acting directly on sensory afferents, γ-motor neurons,
and collateral neurons in the spinal cord to inhibit both
monosynaptic and polysynaptic reflexes. The principal
effect is to reduce the release of excitatory neurotransmitters
by activation of presynaptic GABAB receptors.
This seems to involve a G protein and second-messenger
link that either increases K+ conductance or decreases
Ca++ conductance. |
|
Safety Profile
|
Poison by ingestion,subcutaneous and intravenous routes. Human systemiceffects by ingestion: blood pressure lowering, coma,muscle weakness, pulse rate decrease, respiratorydepression. When heated to decomposition it emits toxicfumes of Cl- |
|
Synthesis
|
Baclofen, 4-amino-3-(4�-chlorophenyl)butyric acid (15.3.5), is synthesized in
two ways. According to the first, 4-chlorobenzaldehyde is condensed with two moles of
acetoacetic ester, giving the product (15.3.1), which initially undergoes alkaline hydrolysis
and decarboxylation forming 3-(4-chlorphenyl)glutaric acid (15.3.2). Dehydration of
this gives 3-(4-chlorophenyl)glutaric acid anhydride (15.3.3), and further treatment with
ammonia gives the corresponding glutarimide (15.3.4). Reacting this with an alkaline solution
of a halogen (Hofmann rearrangement) gives baclofen (15.3.6). |
|
Veterinary Drugs and Treatments
|
Baclofen may be useful to decrease urethral resistance in dogs to
treat urinary retention. It is not recommended for cats. |
|
in vitro
|
(±)-baclofen dampened cell growth in human hepatocellular carcinoma (hcc) cells in a dose-dependent manner. (±)-baclofen also caused cell cycle arrest at g0/g1 phase without inducing cell death. additionally, (±)-baclofen-evoked hcc cells proliferation was associated with down-regulation of the intracellular camp level, up-regulation of p21waf1 protein expression and its phosphorylation level, which could be reversed by pretreatment with the gabab antagonist, phaclofen, indicating that (±)-baclofen-evoked growth blockade was exerted in a gabab-dependent fashion [1]. |
|
in vivo
|
the mice, subcutaneously injected with bel-7402 cells, were given an intraperitoneal injection of (±)-baclofen 30 mg/kg every day for 30 days. compared with the control, (±)-baclofen remarkably blocked the bel-7402 xenograft tumor growth without causing toxic effects via measuring the relative tumor volume and the mean body weight change in (±)-baclofen-treated groups, which could make (±)-baclofen as an effective and relatively safe potential drug for the treatment of hcc [1]. |
|
Drug interactions
|
Potentially hazardous interactions with other drugs
Anti-arrhythmics: enhanced muscle relaxant effect
with procainamide.
Antidepressants: enhanced muscle relaxant effect
with tricyclics.
Antihypertensives: enhanced hypotensive effect.
Lithium: use with caution. |
|
IC 50
|
200 nm: a selective agonist of γ-aminobutyric acid metabotropic receptor (b) (gabab). |
|
Metabolism
|
Baclofen is rapidly and effectively absorbed after oral
administration. It is lipophilic and able to penetrate the
blood-brain barrier.Approximately 35% of the drug is
excreted unchanged in the urine and feces. |
|
references
|
[1]. wang, t., huang, w., & chen, f. baclofen, a gabab receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. life sciences. 2008; 82(9-10): 536-541. |
|
Category
|
toxic substances |
|
Definition
|
ChEBI: A monocarboxylic acid that is butanoic acid substituted by an amino group at position 4 and a 4-chlorophenyl group at position 3. It acts as a central nervous system depressant, GABA agonist and muscle relaxant. |
|
Brand name
|
Kemstro
(Schwarz Pharma); Lioresal (Medtronic); Lioresal (Novartis). |
|
General Description
|
Odorless or practically odorless white to off-white crystalline powder. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego