|
Diuretics
|
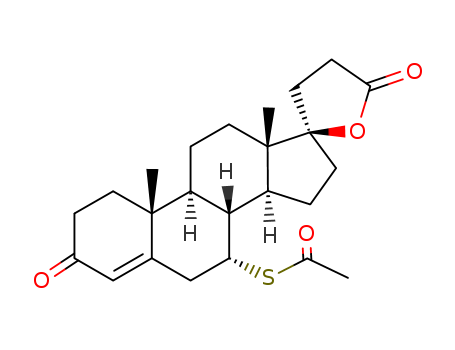
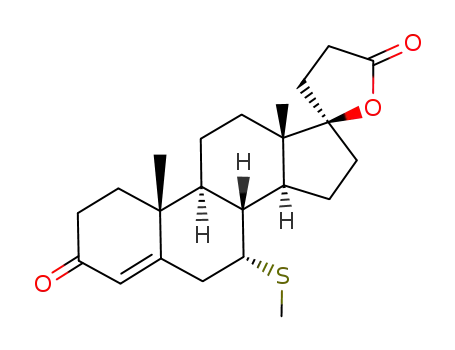
Spironolactone is a kind of weak potassium-sparing diuretics, and is fine white or white-like crystalline powder with slightly bitter taste. It is odorless or with slight mercaptan odor. It is highly soluble in chloroform but insoluble in water and soluble in ethanol, and easily soluble in benzene or ethyl acetate. It has a similar chemical structure as aldosterone and can compete with aldosterone for the aldosterone receptors in the cytoplasm of the distal convoluted tubule and collecting tube duct cells, affecting the binding of the aldosterone to its receptor, thus preventing the synthesis of aldosterone-induced protein and further inhibiting the exchange between K+ and Na+ with reduced potassium excretion and plays potassium-sparing diuretic effect.
The strength of the diuretic effect of spironolactone is related to the in vivo secretion of aldosterone with increased secretion also causing stronger effect while lower secretion causing weaker effects. It has no diuretic effect on patients without aldosterone secretion.
Because the drug only acts on the distal convoluted tubule and collecting duct while having no effect on the other segments of tubular, it only has a weak diuretic effect with slow onset but long duration time. It is mainly used for treating refractory edema accompanied with higher amount of aldosterone such as cirrhosis, congestive heart failure, and nephrotic syndrome with excellent efficacy in treating the patients with hyperaldosteronism caused by the lack of sodium in the congestive heart failure. Single drug administration usually only give a poor diuretic and therefore, it is often used in combination with hydrochlorothiazide or dihydrochlorothiazide for enhancing the effect. In addition, it can also be applied to the treatment of primary hyperaldosteronism and hypertension. Patients of hyperkalemia or renal failure should be disabled.
This product has a rapid and irregular absorption rate after oral administration with the absorption rate being relate to the spironolactone particle size. According to general process, the bioavailability is only about 5% while the absorption rate can reach 90% when made into fine particles with the time for the plasma concentration reaching the peak with approximately 3 hours. It has rapid metabolism rate in vivo with its major metabolites being canrenone which can exert the pharmacological activity and other metabolites also being gradually converted into canrenone. The serum metabolites level will reach peak at 2 to 4 hours after single dose of oral administration. The concentration of metabolites will decrease rapidly within 12 hours with slow decline in 12 to 96 hours. After oral administration of multiple doses, the half life of the canrenone metabolite was 13 to 24 hours. The half-life of spironolactone is only about 10 minutes. The plasma protein binding rate of canrenone is about 98%. Its metabolites can be secreted through the bile and urine. The canrenone metabolism includes enterohepatic circulation. It can penetrate through the placental barrier and enter into breast milk. It also has effects of inhibiting estrogen and androgen synthesis. |
|
Indications
|
Spironolactone is a kind of competitive aldosterone inhibitors with the drug itself being not active. Its diuretic effect is through its competitive antagonism against endogenous aldosterone, belonging to potassium-sparing diuretics.
1. edema disease, being used in combination other diuretics for the treatment of congestive heart failure, liver cirrhosis, renal edema, edema disease; the purpose in to correct the increased secondary aldosterone secretion associated with those above diseases and fight against the potassium excretion of other diuretics; it can also used in the treatment of idiopathic edema.
2. Hypertension, as the auxiliary treatment drug of high blood pressure.
3. For primary hyperaldosteronism, spironolactone can be used to diagnose and treat the disease.
4. For the prevention of hypokalemia; being used in combination with thiazide diuretics to enhance the diuretic effect and prevent hypokalemia.
The above information is edited by the lookchem of Dai Xiongfeng. |
|
Toxicity grading
|
highly toxic |
|
Acute toxicity
|
intraperitoneal-rat LD50: 277 mg/kg; intraperitoneal port-Mouse LD50: 260 mg/kg |
|
Flammability and hazardous characteristics
|
easily flammable with combustion generating toxic fumes of sulfur oxides |
|
Storage characteristics
|
Treasury: ventilation, low-temperature and dry; store it separately from food raw materials |
|
Extinguishing agent
|
Dry powder foam, sand, carbon dioxide, spray water |
|
Manufacturing Process
|
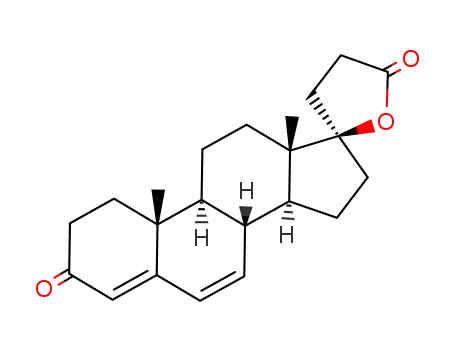
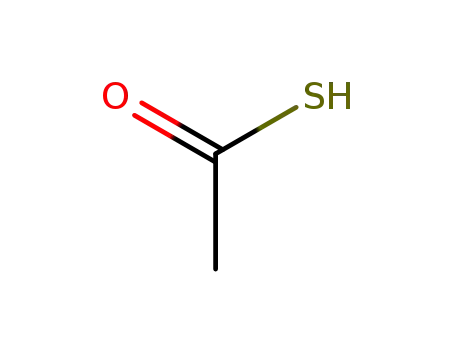
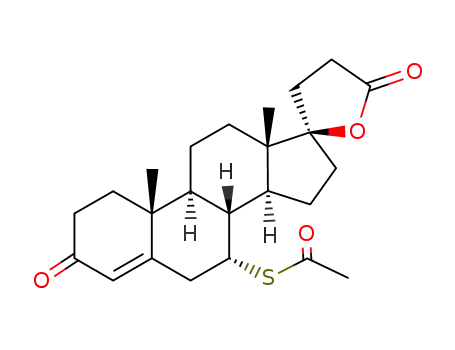
A mixture of approximately 11 parts of 17α-(2-carboxyethyl)-17β-
hydroxyandrosta-4,6-dien-3-one lactone and 10 parts of thioacetic acid is
heated at 85° to 95°C for ? hour. Excess thioacetic acid is removed by
vacuum distillation at this point, and the residue is twice recrystallized from
methanol, affording 7α-acetylthio-17α-(2-carboxyethyl)-17β-hydroxyandrost-
4-en-3-one lactone, melting at approximately 134° to 135°C. Heated above
this melting point, the product solidifies and melts again at approximately
201° to 202°C (with decomposition). |
|
Therapeutic Function
|
Diuretic |
|
World Health Organization (WHO)
|
Spironolactone, an aldosterone antagonist, has been widely used
for over 25 years in the treatment of hypertension and in the management of
refractive oedema. Evidence that long-term administration of high doses are
tumorigenic in the rat has recently led to restriction of its use by some national
regulatory authorities although the significance of this finding with respect to
clinical use is not certain. In 1987 spironolactone was transferred from the main list
to the complementary list of the WHO Model List of Essential Drugs. (See also WHO comments for canrenone and potassium canrenoate).
(Reference: (WHODI) WHO Drug Information, 2(1), , 1988) |
|
Biological Functions
|
Spironolactone (Aldactone) is structurally related to
aldosterone and acts as a competitive inhibitor to prevent
the binding of aldosterone to its specific cellular binding
protein. Spironolactone thus blocks the hormone-induced
stimulation of protein synthesis necessary for Na+ reabsorption
and K+ secretion. Spironolactone, in the presence
of circulating aldosterone, promotes a modest increase in
Na+ excretion associated with a decrease in K+ elimination.
The observations that spironolactone is ineffective in
adrenalectomized patients and that the actions of
spironolactone can be reversed by raising circulating al-dosterone blood levels (surmountable antagonism) support
the conclusion that spironolactone acts by competitive
inhibition of the binding of aldosterone with receptor
sites in the target tissue. Spironolactone acts only when
mineralocorticoids are present. |
|
Hazard
|
Questionable carcinogen. |
|
Biological Activity
|
Competitive mineralocorticoid (aldosterone) receptor antagonist that exhibits antihypertensive activity in vivo . Also displays antiandrogen activity and inhibits steroid hormone biosynthesis. |
|
Biochem/physiol Actions
|
Spironolactone is a competitive aldosterone receptor antagonist. Used as potassium sparing diuretic. |
|
Mechanism of action
|
Spironolactone is a potassium sparing diuretic that has a different mechanism of action
than other drugs of this class.
It is a competitive antagonist of aldosterone, and its action is most effective when the
level of circulated aldosterone in the organism is high. |
|
Pharmacology
|
Spironolactone (Aldactone) is the only diuretic that has
been shown in a double-blind multicenter prospective
clinical trial to improve survival in CHF. The addition
of spironolactone to digitalis and an angiotensinconverting
enzyme (ACE) inhibitor significantly improved
survival among patients with chronic severe
heart failure.
Spironolactone competitively inhibits the binding of
aldosterone to cytosolic mineralocorticoid receptors in
the epithelial cells in the late distal tubule and collecting duct of the kidney. Aldosterone enhances salt and
water retention at the expense of enhanced renal K
and H excretion. Spironolactone enhances diuresis by
blocking sodium and water retention while retaining
potassium. An obvious potential side effect is hyperkalemia,
which is aggravated by the potassium-retaining
properties of the ACE inhibitors. The likely concomitant
use of the loop diuretic furosemide, which depletes
K , dictates careful monitoring of serum potassium to
avoid life-threatening rhythm disturbances.
There is also evidence for the existence of mineralocorticoid
receptors on cardiac myocytes. This raises the
intriguing possibility that spironolactone could mediate
important direct effects on the myocardium in CHF. |
|
Side effects
|
Serum electrolyte balance should be monitored periodically,
since potentially fatal hyperkalemia may occur,especially in patients with impaired renal function or excessive
K+ intake (including the K+ salts of coadministered
drugs, e.g., potassium penicillin). Spironolactone
can induce hyponatremia and in cirrhotic patients, metabolic
acidosis.A variety of gastrointestinal disturbances
may accompany spironolactone administration. These
include diarrhea, gastritis, gastric bleeding, and peptic ulcers.
Spironolactone is contraindicated in patients with
peptic ulcers. Spironolactone may also cause elevated
blood urea nitrogen, drowsiness, lethargy, ataxia, confusion,
and headache. Gynecomastia and menstrual irregularity
in males and females, respectively, can occur.
Painful gynecomastia (directly related to dosage level
and duration of therapy), which is generally reversible,
may necessitate termination of therapy. Animal studies
demonstrating tumorigenic potential support the clinical
judgment that spironolactone alone or in combination
should not be used for most patients who require diuretic
therapy and its unnecessary use should be
avoided. |
|
Safety Profile
|
Poison by intraperitoneal route. Human reproductive effects by ingestion and possibly other routes: men, impotence and breast development; women, menstrual cycle changes or disorders, changes in the breasts and lactation. An experimental teratogen. Other experimental reproductive effects. Other human systemic effects by ingestion: agranulocytosis, kidney tubule damage, increased urine volume, and changes in blood sodium and calcium levels. Questionable carcinogen. When heated to decomposition it emits toxic fumes of SOx,. Used to treat hypertension, edema of congestive heart failure, cirrhosis, and kidney failure.
0 |
|
Synthesis
|
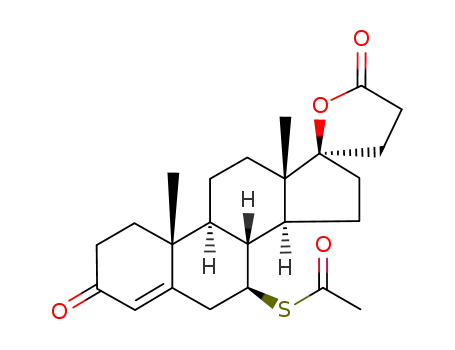
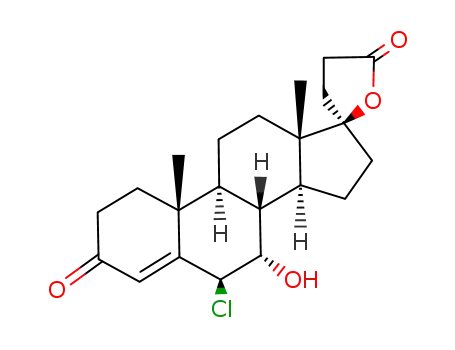
Spironolactone is the 7-acetate of the γ-lactone of 17-hydroxy-7-mercapto-
3-oxo-17-α-pregn-4-ene-21-carboxylic acid (21.5.8). Spironolactone is synthesized industrially
in two different ways from androstenolone—3β-hydroxy-5-androsten-17-one.
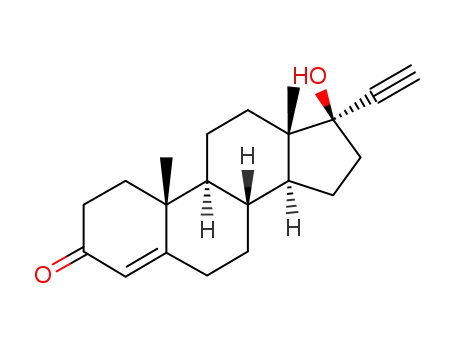
According to the first method, androstenolone undergoes ethynylation by acetylene in a
Normant reaction condition using sodium amide in liquid ammonia, which forms 17
α-ethynyl-3β-,17β-dihydroxy-5-androstene (21.5.1). Subsequent reaction of this with
methylmagnesiumbromide and then with carbon dioxide gives the corresponding propenal
acid (21.5.2). Reduction of the triple bond in this product with hydrogen using a palladium
on calcium carbonate catalyst forms the corresponding acrylic acid derivative (21.5.3), which
is treated with acid without being isolated, which leads to cyclization into an unsaturated lactone
derivative (21.5.4). The double bond is reduced by hydrogen, in this case using a palladium
on carbon catalyst. The resulting lactone 21.5.5 undergoes oxidation in an Oppenauer
reaction, giving an unsaturated keto-derivative—4-androsten-3,17-dione (21.5.6). Further
oxidation of the product (21.5.6) using chloroanyl gives dienone (21.5.7), which when
reacted with thioacetic acid gives the desired spionolactone (21.5.8). |
|
Veterinary Drugs and Treatments
|
Spironolactone may be used in patients with congestive heart failure
who do not adequately respond to furosemide and ACE inhibitors,
who develop hypokalemia on other diuretics, and are unwilling or
unable to supplement with exogenous potassium sources. It may
also be effective in treating ascites as it has less potential to increase
ammonia levels than other diuretics. |
|
Drug interactions
|
Potentially hazardous interactions with other drugs
ACE inhibitors or angiotensin-II antagonists:
enhanced hypotensive effect; risk of severe
hyperkalaemia.
Antibacterials: avoid with lymecycline.
Antidepressants: increased risk of postural
hypotension with tricyclics.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect with
post-synaptic alpha-blockers.
Cardiac glycosides: increased digoxin concentration.
Ciclosporin: increased risk of hyperkalaemia.
Cytotoxics: avoid with mitotane; increased risk
of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium: reduced lithium excretion.
NSAIDs: increased risk of hyperkalaemia (especially
with indometacin); increased risk of nephrotoxicity;
diuretic effect of spironolactone antagonised by
aspirin.
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia. |
|
Metabolism
|
Spironolactone is poorly absorbed after oral administration
and has a delayed onset of action; it may take several
days until a peak effect is produced. It has a somewhat
slower onset of action than triamterene and
amiloride (discussed later), but its natriuretic effect is
modestly more pronounced, especially during long-term
therapy. Spironolactone is rapidly and extensively metabolized,
largely to the active metabolite canrenone.
Canrenone and potassium canrenoate, its K+ salt, are
available for clinical use in some countries outside the
United States. Canrenone has a half-life of approximately
10 to 35 hours.The metabolites of spironolactone are excreted
in both the urine and feces. New selective aldosterone
receptor antagonists (SARA), such as eplerenone,
have been developed but have not yet been introduced
into clinical practice. Eplerenone and canrenone exhibit
fewer steroidlike side effects (gynecomastia, hirsutism). |
|
Structure and conformation
|
Synthetic steroid that resembles aldosterone. |
|
Category
|
toxic substances |
|
Definition
|
ChEBI: A steroid lactone that is 17alpha-pregn-4-ene-21,17-carbolactone substituted by an oxo group at position 3 and an alpha-acetylsulfanyl group at position 7. |
|
Brand name
|
Aldactone (Searle);Airolactone;Aldactide 25;Aldactone-a;Aldazida;Aldonorm;Aldospirone;Alpamed;Altexide;Aporasnon;Carditan;Crk 635;Ct-spiro;Digi-aldopur;Dilakton;Hexalacton;Hokulaton;Hokuraton;Hydrospiron;Idrolattone;Lacilactone;Laralmin;Lasitone;Loractone;Mf 218d;Noidouble;Novospiroton;Novospirozine;Novosprioton;Novospriozine;Penantin;Pirolacton;Pirolcaton;Plarenil;Practon 50;Raudazida;Risicordin;Rolactone microfine;Sali-spiroctan;Sas 1060;Servilactone;Spiractin;Spiridazide;Spirix;Spiro comp;Spiro50-d;Spirodigital;Spiro-f;Spironomocompren;Spironone;Spironothiazide;Spiropal;Spirostada;Spirotone;Suprapuren;Synureticum;Tensoflex;Urosonine;Xenalone;Xeualon. |
|
General Description
|
Spironolactone, 7α-(acetylthio)-17α-hydroxy-3-oxopregn-4-ene-3-one-21-carboxylic acidγ-lactone (Aldactone) is an aldosterone antagonist of greatmedical importance because of its diuretic activity. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego