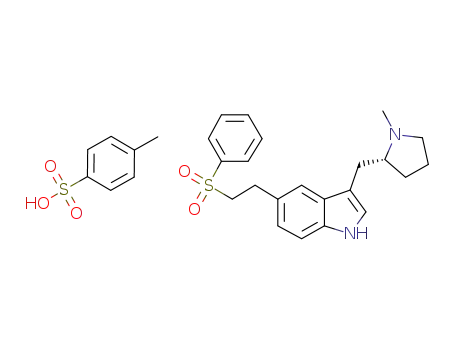
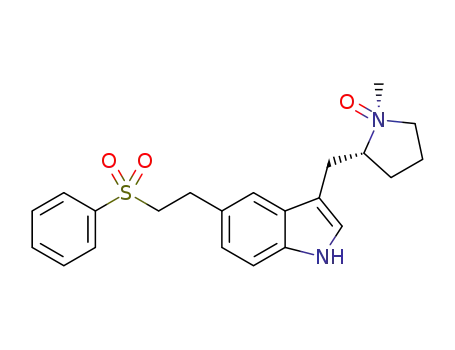
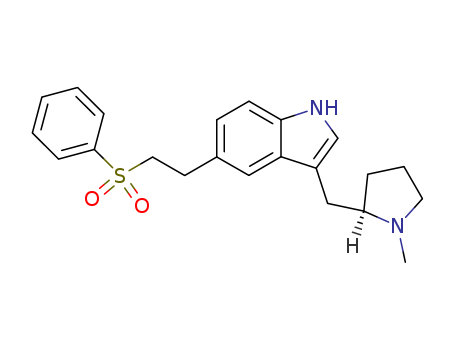
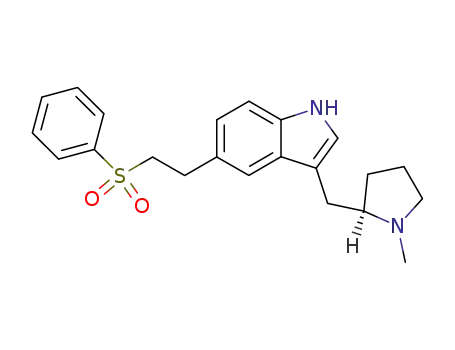
Manufacturer supply top purity Eletriptan 143322-58-1 with ISO standards
- Molecular Formula:C22H26N2O2S
- Molecular Weight:382.527
- Appearance/Colour:yellow foam
- Vapor Pressure:1.58E-16mmHg at 25°C
- Boiling Point:613.4 °C at 760 mmHg
- PKA:17.14±0.30(Predicted)
- Flash Point:324.8 °C
- PSA:61.55000
- Density:1.235 g/cm3
- LogP:4.83970
Eletriptan(Cas 143322-58-1) Usage
|
Manufacturing Process
|
A mixture of the appropriate phenyl vinyl sulfone, tri-o-tolylphosphine,
palladium (II) acetate, triethlamine and (R)-5-bromo-3-(N�methylpyrrolidinylmethyl)-1H-indole in anhydrous acetonitrle was heated at
reflux under nitrogen. The resultant reaction mixture was evaporated under
reduced pressure, and the residue was column chromatographed using silica
gel and elution with methylene chloride/absolute ethanol/ammonia to afford
the (R )-5-trans-(2-phenylsulfonylethenyl)-3-(N-methylpyrrolidin-2-ylmethyl)-
1H-indole.A solution of (R)-5-trans-(2-phenylsulfonylethenyl)-3-(N-methylpyrrolidin-2-
ylmethyl)-1H-indole and 10% Pd/C in ethanolic hydrogen chloride (prepared
from absolute ethanol and acetyl chloride and N,N-dimethylformamide was
shaken under a hydrogen atmosphere at room temperature). The resultant
reaction mixture was filtered through diatomaceous earth (Celite trademark),
washed with absolute ethanol, and the combined filtrates were evaporated
under reduced pressure. The residue was partitioned between ethyl acetate
and water. The organic phase was separated, washed with water, brine, dried(Na2SO4), and evaporated under reduced pressure to afford a oil product.
Column chromatography of this product using silica gel and elution with
methylene chloride/absolute ethanol/ammonia afforded the appropriate (R)-5-
(2-phenylsulfonylethyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole.The salt eletriptan hydrobromide may be produced by reaction of the (R)-5-
(2-phenylsulfonylethyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole with
hydrobromic acid. |
|
Therapeutic Function
|
Serotonin agonist |
|
Drug interactions
|
Potentially hazardous interactions with other drugsAntibacterials: concentration increased by
clarithromycin and erythromycin - avoid.Antidepressants: increased risk of CNS toxicity with
citalopram - avoid; possibly increased serotonergic
effects with duloxetine and venlafaxine; increased
serotonergic effects with St John’s wort - avoidAntifungals: concentration increased by itraconazole
and ketoconazole - avoid.Antivirals: concentration increased by indinavir and
ritonavir - avoid.Dapoxetine: possible increased risk of serotonergic
effects - avoid for 2 weeks after stopping 5HT1
agonistsErgot alkaloids: increased risk of vasospasm - avoid. |
|
Metabolism
|
In vitro studies indicate that eletriptan is primarily
metabolised by hepatic cytochrome P-450 enzyme
CYP3A4. This finding is substantiated by increased
plasma concentrations of eletriptan followingknown selective and potent CYP3A4 inhibitors. In vitro
studies also indicate a small involvement of CYP2D6
although clinical studies do not indicate any evidence of
polymorphism with this enzyme.There are two major circulating metabolites identified
that significantly contribute to plasma radioactivity
following administration of 14C-labelled eletriptan. The
metabolite formed by N-oxidation, has demonstrated no
activity in animal in vitro models. The metabolite formed
by N-demethylation, has been demonstrated to have
similar activity to eletriptan in animal in vitro models.
A third area of radioactivity in plasma has not been
formally identified, but is most likely to be a mixture of
hydroxylated metabolites which have also been observed
excreted in urine and faeces.The plasma concentrations of the N-demethylated
active metabolite are only 10-20% of those of parent,
so would not be expected to significantly contribute to
the therapeutic action of eletriptan. Non-renal clearance
accounts for approximately 90% of the total clearance
indicating that eletriptan is eliminated primarily by
metabolism. |
|
Definition
|
ChEBI: Eletriptan is an N-alkylpyrrolidine, being N-methylpyrrolidine in which the pro-R hydrogen at position 2 is substituted by a {5-[2-(phenylsulfonyl)ethyl]-1H-indol-3-yl}m
thyl group. |
|
Brand name
|
Relpax |
|
General Description
|
Eletriptan, introduced into the market in 2002, is the newesttriptan with highest affinity for 5-HT1B, 5-HT1D, and 5-HT1Freceptors. It is one of the most lipophilic triptans marketedto date and is well tolerated and safe across its dosing rangeof 20 to 80 mg. However, it is metabolized primarily(>90%) by CYP3A4 isozyme to its active metabolite, theN-desmethyleletriptan, which accounts for approximately10% to 20% of the plasma concentration of that observedfor parent drug. Thus, coadministration of eletriptan withpotent CYP3A4 inhibitors such as ketoconazole, itraconazole,nefazodone, troleandomycin, clarithromycin, ritonavir,and nelfinavir may require dose reduction and closer monitoringfor CNS side effects. Furthermore, becauseeletriptan and its active metabolite, N-desmethyleletriptan,are also substrates for the P-glycoprotein efflux pumps thatare responsible for their removal from the brain, coadministrationof eletriptan with a known P-glycoprotein inhibitorand/or inducer such as digoxin, diltiazem, verapamil, or St.John’s Worth would result in higher brain levels of its activemetabolite, and thus a higher rate of the CNS side effectsreported for this drug. |
InChI:InChI=1/C22H26N2O2S.BrH/c1-24-12-5-6-19(24)15-18-16-23-22-14-17(9-10-21(18)22)11-13-27(25,26)20-7-3-2-4-8-20;/h2-4,7-10,14,16,19,23H,5-6,11-13,15H2,1H3;1H/t19-;/m1./s1
143322-58-1 Relevant articles
Investigational study into the formation of methoxy derivative and other impurities during the optimization of eletriptan hydrobromide
Kumar, U. Sampath,Sankar, V. Ravi,Rao, M. Malleswara,Jaganathan,Buchi Reddy
, p. 1917 - 1920 (2012)
During the process development of eletri...
A Low Rhodium Content Smart Catalyst for Hydrogenation and Hydroformylation Reactions
Paganelli, Stefano,Tassini, Riccardo,Rathod, Vikas D.,Onida, Barbara,Fiorilli, Sonia,Piccolo, Oreste
, p. 1508 - 1521 (2020/10/15)
Abstract: This paper describes the prepa...
PROCESS FOR PREPARING (( R)-3-[(-1-METHYLPYRROLIDIN-2-YL)METHYL]-5-(2-PHENYLSULFONYLETHYL)-1H-INDOLE
-
, (2017/09/27)
The present invention provides an effici...
A synthesis method according to sets up qu tan hydrobromide
-
Paragraph 0061-0063, (2017/10/22)
The invention discloses a synthesis meth...
A NOVEL PROCESS FOR THE PREPARATION OF ELETRIPTAN
-
Paragraph 0033, (2013/07/25)
The present invention relates to a novel...
143322-58-1 Process route
-
-
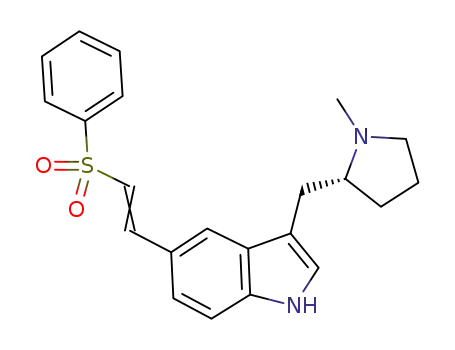
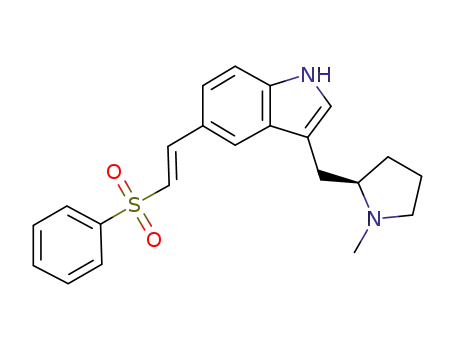
(R)-5-(2-Benzenesulphonylethenyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole
-
-
209682-64-4
(R)-3-[(1-methyl-2-pyrrolidinyl)-methyl]-5-ethyl-1H-indole
Conditions
| Conditions |
Yield |
|
With
hydrogen;
In
methanol; toluene;
at 80 ℃;
for 0.5h;
under 3750.38 Torr;
Temperature;
Pressure;
Overall yield = 84 percent;
Inert atmosphere;
Schlenk technique;
Autoclave;
|
84%
16%
|
-
-
1225327-17-2
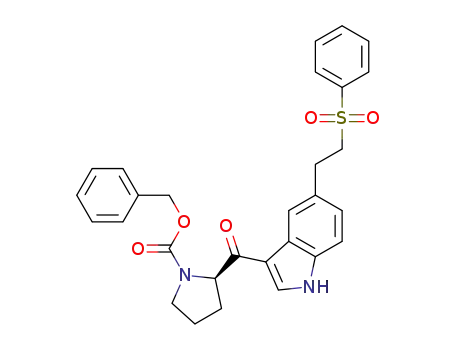
(R)-benzyl 2-(5-(2-(phenylsulfonyl)ethyl)-1H-indole-3-carbonyl)pyrrolidine-1-carboxylate
Conditions
| Conditions |
Yield |
|
(R)-benzyl 2-(5-(2-(phenylsulfonyl)ethyl)-1H-indole-3-carbonyl)pyrrolidine-1-carboxylate;
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
Reflux;
With
water; sodium hydroxide;
In
tetrahydrofuran;
at 0 - 5 ℃;
|
80%
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
Reflux;
|
80%
|
|
Multi-step reaction with 2 steps
1: palladium 10% on activated carbon; hydrogen; methanesulfonic acid / acetone / 2327.23 Torr
2: lithium aluminium tetrahydride / tetrahydrofuran / 0 °C / Reflux
With
lithium aluminium tetrahydride; methanesulfonic acid; palladium 10% on activated carbon; hydrogen;
In
tetrahydrofuran; acetone;
|
|
143322-58-1 Upstream products
-
180637-89-2
(R)-5-[(2-phenylsulfonyl)ethenyl]-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole
-
75-75-2
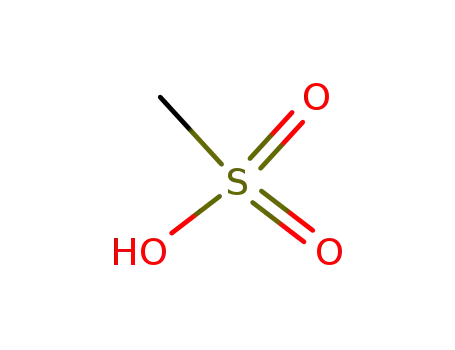
methanesulfonic acid
-
868618-81-9
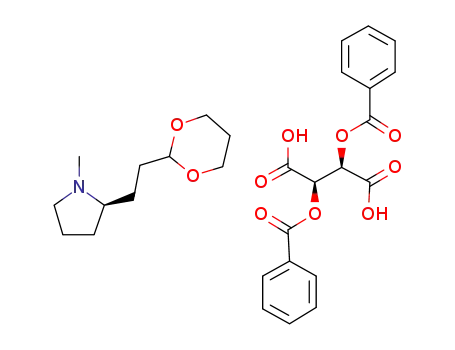
(2R)-2-[2-(1,3-dioxan-2yl)ethyl]-1-methylpyrrolidine (2R,3R)-2,3-bis(benzyloxy)succinic acid
-
1162655-06-2
3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate
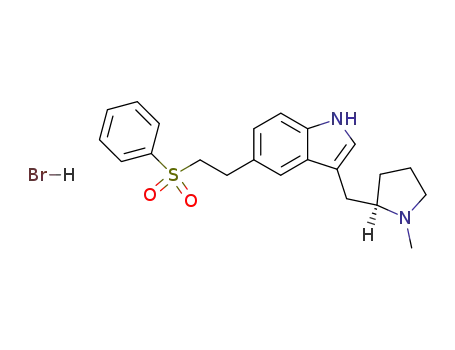
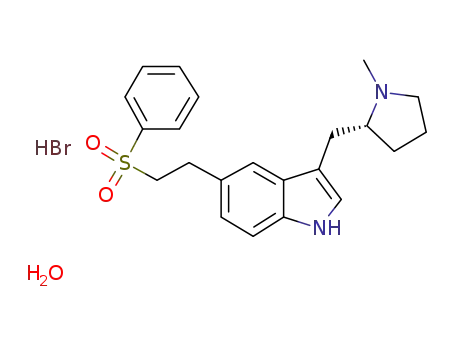
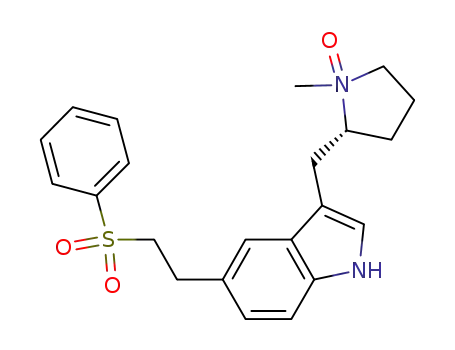
143322-58-1 Downstream products
-
177834-92-3
eletriptan hydrobromide
-
273211-28-2
3-(N-methyl-2(R)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1H-indole hydrobromide monohydrate
-
1217641-89-8
(R)-5-[2-(phenylsulfonyl)ethyl]-3-[(1-methyl-2-pyrrolidinyl-N-oxide)methyl]-1H-indole
-
1408337-87-0
(1S,2R)-3-[(1-methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-N-oxide-1H-indole
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego



![(R)-3-[(1-methyl-2-pyrrolidinyl)-methyl]-5-ethyl-1H-indole](/upload/2025/3/cee28f9d-35e3-4443-8836-289ab6330834.png)