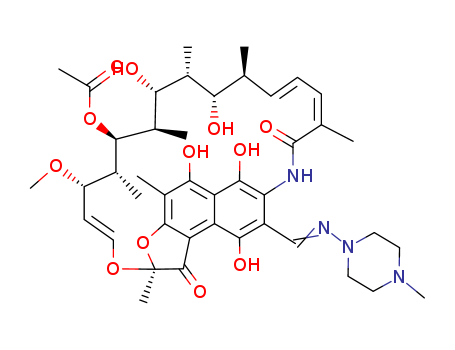
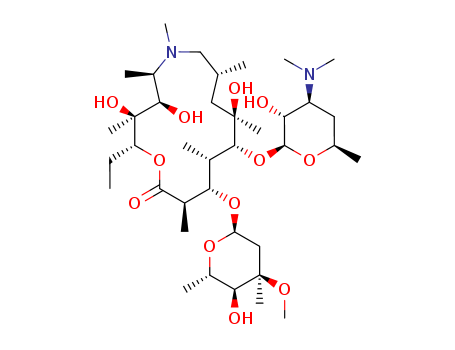
Top Quality Chinese Manufacturer supply 13292-46-1 Rifampicin We supply high quality Rifampicin (CAS 13292-46-1), in stock, factory directly supply to clients, lower prices, more competitiveness.
What is the Rifampicin ?
Rifampicin is Red to orange crystalline solid, while it's Molecular Formula is C43H58N4O12. Rifampicin is used to treat Tuberculosis and Tuberculosis-related mycobacterial infections. It is widely used as an antipruritic agent in the autoimmune cholestatic liver disease, primary biliary cirrhosis (PBC). It has been shown to cause hepatitis.
What is the CAS number for Rifampicin ?
The CAS number of Rifampicin is 13292-46-1.
More information of Rifampicin 13292-46-1 are:
|
Synonyms
|
3-[[(4-methyl-1-piperazinyl)imino]methyl]-rifamycin;3-(((4-Methyl-1-piperazinyl)imino)methyl)rifamycin SV;5,6,9,17,19,21-Hexahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-8-(N-(4-methyl-1-piperazinyl)formimidoyl)-2,7-(epoxypentadeca(1,11,13)trienimino)naphtho(2,1-b)furan-1,11(2H)-dione 21-acetate;8-(4-Methylpiperazinyliminomethyl) rifamycin SV; |
|
CAS Number
|
13292-46-1 |
|
Molecular Formula
|
C43H58N4O12 |
|
Molecular Weight
|
822.953 |
|
Density
|
1.34 g/cm3 |
|
Melting Point
|
183°C (dec.) |
|
Boiling Point
|
1004.42 °C at 760 mmHg |
|
Flash Point
|
561.253 °C |
|
HS CODE
|
3003.20
|
|
PSA
|
220.15000 |
|
LogP
|
4.34920 |
|
Pka
|
1.7, 7.9(at 25℃) |
What is Rifampicin (13292-46-1) used for?
Rifampicin is a semisynthetic derivative of rifamicin B, a macrolactam antibiotic and one
of more than five antibiotics from a mixture of rifamicins A, B, C, D, and E, which is called
a rifamicin complex, which is produced by actinomycetes Streptomyces mediteranei
(Nocardia mediteranei). It was introduced into medical practice in 1968. Synthesis of
rifampicin begins with an aqueous solution of rifamicin, which under the reaction conditions
is oxidized to a new derivative of rifamicin S (32.7.4), with the intermediate formation of rifamicin O (32.7.3). Reducing the quinone structure of this product with hydrogen using a
palladium on carbon catalyst gives rifamicin SV (32.7.5). The resulting product undergoes
aminomethylation by a mixture of formaldehyde and pyrrolidine, giving 3-pyrrolidi�nomethylrifamicin SV (32.7.6). Oxidizing the resulting product with lead tetracetate to an
enamine and subsequent hydrolysis with an aqueous solution of ascorbic acid gives
3-formylrifamicin SV (32.7.7). Reacting this with 1-amino-4-methylpiperazine gives the
desired rifampicin (32.7.8).
InChI:InChI=1/C43H58N4O12/c1-21-12-11-13-22(2)42(55)45-33-28(20-44-47-17-15-46(9)16-18-47)37(52)30-31(38(33)53)36(51)26(6)40-32(30)41(54)43(8,59-40)57-19-14-29(56-10)23(3)39(58-27(7)48)25(5)35(50)24(4)34(21)49/h11-14,19-21,23-25,29,34-35,39,49-53H,15-18H2,1-10H3,(H,45,55)/b12-11+,19-14+,22-13-,44-20?/t21?,23-,24-,25-,29+,34+,35-,39+,43+/m1/s1
Articles related to Rifampicin:
|
Article
|
Source
|
|
Antitubercular nanocarrier combination therapy: Formulation strategies and in vitro efficacy for rifampicin and SQ641
|
Daddio, Suzanne M.,Reddy, Venkata M.,Liu, Ying,Sinko, Patrick J.,Einck, Leo,Prudhomme, Robert K.
, p. 1554 - 1563 (2015)
|
|
Method of purifying rifampicin
|
-
Paragraph 0056-0060, (2020/05/01)
|
How to get the best price on Rifampicin?
weifang yangxu group co.,ltd is a quality supplier and manufacturer of Rifampicin . You can buy high quality, low price Rifampicin 13292-46-1 here. Contact us.
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego