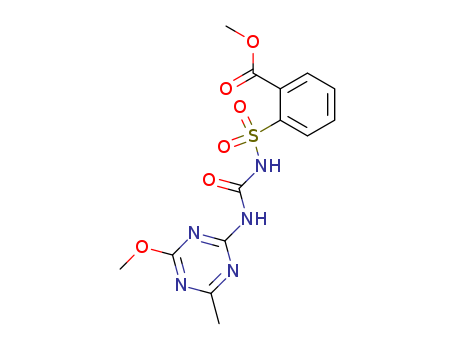
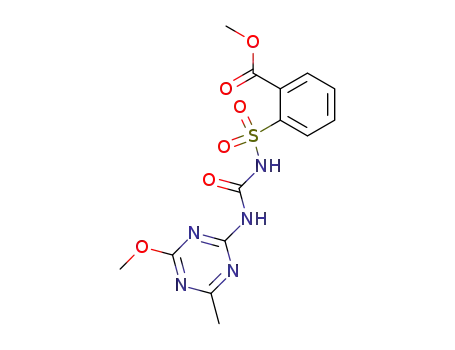
Reputable factory supply Agrochemicals Herbicide Metsulfuron-Methyl 74223-64-6 in stock with high standard
- Molecular Formula:C14H15N5O6S
- Molecular Weight:381.369
- Appearance/Colour:faint, sweet ester-like white to pale yellow solid
- Vapor Pressure:1.23E-17mmHg at 25°C
- Melting Point:158 °C
- Refractive Index:1.593
- Boiling Point:647.2 °C at 760 mmHg
- PKA:2.55±0.10(Predicted)
- Flash Point:345.2 °C
- PSA:157.85000
- Density:1.473 g/cm3
- LogP:2.03030
2-[[[[(4-Methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoic acid methyl ester(Cas 74223-64-6) Usage
|
Trade name
|
ALLIE?; ALLY?; ALLY-20DF?; ANSWER?;
BRUSH-OFF?; CANVAS?; CIMARRON?; DMC? WEED
CONTROL; DPD 63760H?; DPX 6376?; DPX-T 6376?;
ESCORT?; FINESSE?; GROPPER?; NUP?; PARTISAN
?; PASTURE? MD; RIVERDALE?; ROSULFURON? |
|
Potential Exposure
|
Metsulfuron-methyl is a preemergence
and postemergence sulfonylurea herbicide used to control
annual grasses, brush, woody plants and broadleaf weeds. It
can be applied to cereals including barley, rye and wheat
and to pastures. It is primarily used to control brush, woody
plants and broadleaf weeds on rights-of-way, fence rows,
storage areas, highways and other noncrop areas. |
|
Environmental Fate
|
Soil/Plant. Hydrolyzes in soil and plants to nontoxic products (Hartley and Kidd,
1987). The half-life in soil varies from 7 days to 1 month (Hartley and Kidd, 1987).
Ismail and Lee (1995) studied the persistence of metsulfuron-methyl in a sandy loam
(pH 5.1) and clay soil (3.1) under laboratory conditions. Degradation was more rapid in
non-sterilized than in sterilized soil. In non-sterilized soil, the rate of degradation increased
with increasing soil moisture content. When the moisture level in the sandy loam and clay
soil was increased from 20 to 80% of field capacity at 35°C, the half-lives were reduced
from 9.0 to 5.7 and 11.2 to 4.6, days, respectively. The investigators concluded that the
disappearance of metsulfuron-methyl in soil resulted from microbial degradation and
chemical hydrolysis. |
|
Metabolic pathway
|
In soils, under aerobic conditions, metsulfuron methyl
is degraded by the cleavage of the sulfonylurea
linkage, resulting in the formation of methyl 2-
(aminosulfonyl)benzoate, 4-methoxy-6-methyl-2-amino-
1,3,5-triazine, and saccharin as major products. Under
anaerobic conditons, free acid of metsulfuron methyl
and the resulting O-demethylation product are
identified. The formation of two ring-opening products at the triazine moiety is observed. Under acidic
conditions, hydrolytic degradation products identified
are involved in the soil degradation products. In plants,
the specific metabolites are identified as the
hydroxylation product of the phenyl ring of metsulfuron
methyl and 4-methoxy-6-hydroxymethyl-2-amino-1,3,5-
triazine. Mammalian metabolites are also included in
the soil metabolites. |
|
Incompatibilities
|
Strong oxidizers. Stable in air to about
140 C. It is hydrolyzed in acid solutions. |
|
Waste Disposal
|
It is the responsibility of
chemical waste generators to determine the toxicity and
physical properties and of a discarded chemical and to
properly identify its classification and certification as a hazardous
waste and to determine the disposal method. United
States Environmental Protection Agency guidelines for the
classification determination are listed in 40 CFR Parts
261.3. In addition, waste generators must consult and follow
all regional, national, state and local hazardous waste
laws to ensure complete and accurate classification and disposal
methods. Recycle any unused portion of the material
for its approved use or return it to the manufacturer or
supplier. Offer surplus and nonrecyclable solutions to a
licensed disposal company. Dissolve or mix the pesticide
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. |
|
Definition
|
ChEBI: A N-sulfonylurea in which the sulfonyl group is attached to a 2-(methoxycarbonyl)phenyl group while a (4-methoxy-6-methyl-1,3,5-triazin-2-yl group replaces one of the amino hydrogens of the remaining urea group. |
|
Agricultural Uses
|
Herbicide: Metsulfuron-methyl is a pre-emergence and
post-emergence herbicide used to control annual grasses,
brush, woody plants and broadleaf weeds. It can be applied
to cereals including barley, rye and wheat and to pastures.
It is primarily used to control brush, woody plants
and broadleaf weeds on rights-of-way, fence rows, storage
areas, highways and other non-crop areas. |
InChI:InChI=1/C14H15N5O6S/c1-8-15-12(18-14(16-8)25-3)17-13(21)19-26(22,23)10-7-5-4-6-9(10)11(20)24-2/h4-7H,1-3H3,(H2,15,16,17,18,19,21)
74223-64-6 Relevant articles
PROCESS FOR PREPARING A NOVEL CRYSTALLINE FORM OF METSULFURON-METHYL AND USE OF THE SAME
-
Paragraph 0062, (2017/05/19)
A crystalline form of metsulfuron-methyl...
Halopyridyl triazolinone herbicides and herbicidal use thereof
-
, (2008/06/13)
Disclosed are herbicidal halopyridyl tri...
N-phenylpyrrolidines
-
, (2008/06/13)
The 1-(3,5-bis-trifluoromethylphenyl)-2-...
Herbicidal compound concentrate
-
, (2008/06/13)
There is provided a novel herbicidal com...
74223-64-6 Process route
-
-
1668-54-8
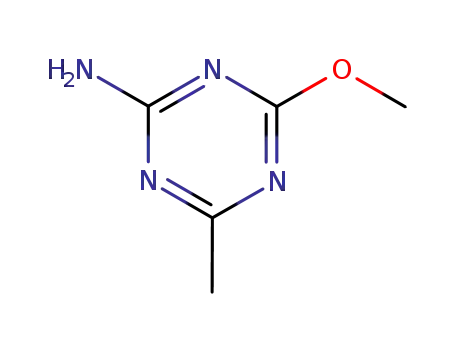
4-methoxy-6-methyl-1,3,5-triazin-2-amine
-
-
26638-43-7
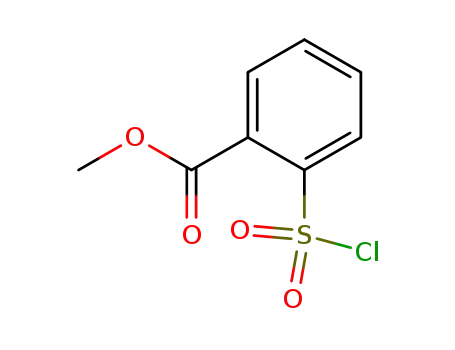
2-methoxycarbonylbenzenesulfonyl chloride
-
-
74223-64-6
Metsulfuron-methyl
Conditions
| Conditions |
Yield |
|
In
1-methyl-pyrrolidin-2-one;
|
55%
|
-
-
74222-95-0
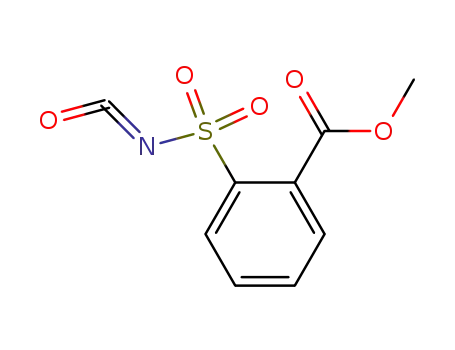
2-(methoxycarbonyl)benzenesulfonyl isocyanate
-
-
1668-54-8
4-methoxy-6-methyl-1,3,5-triazin-2-amine
-
-
74223-64-6
Metsulfuron-methyl
Conditions
| Conditions |
Yield |
|
In
dichloromethane;
|
|
|
In
dichloromethane;
at 20 ℃;
for 16h;
|
|
74223-64-6 Upstream products
-
1668-54-8
4-methoxy-6-methyl-1,3,5-triazin-2-amine
-
26638-43-7
2-methoxycarbonylbenzenesulfonyl chloride
-
74222-95-0
2-(methoxycarbonyl)benzenesulfonyl isocyanate
74223-64-6 Downstream products
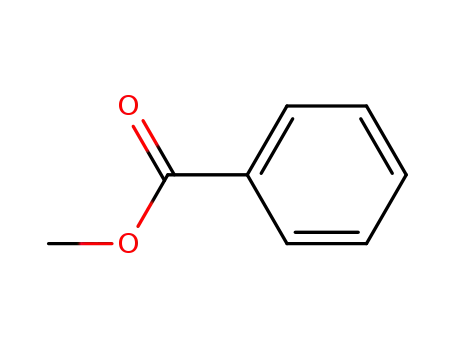
-
93-58-3
benzoic acid methyl ester
-
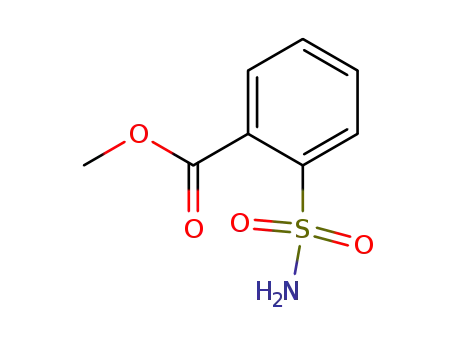
57683-71-3
methyl 2-(aminosulfonyl)benzoate
-
74222-95-0
2-(methoxycarbonyl)benzenesulfonyl isocyanate
-
1668-54-8
4-methoxy-6-methyl-1,3,5-triazin-2-amine
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego