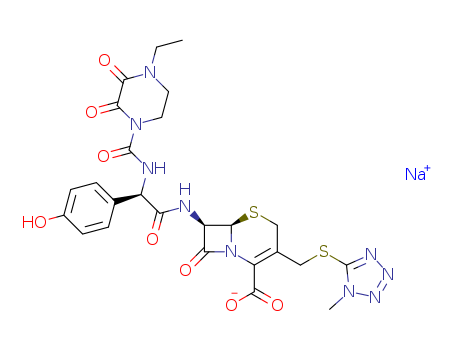
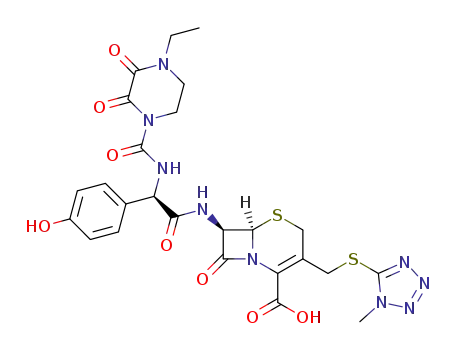
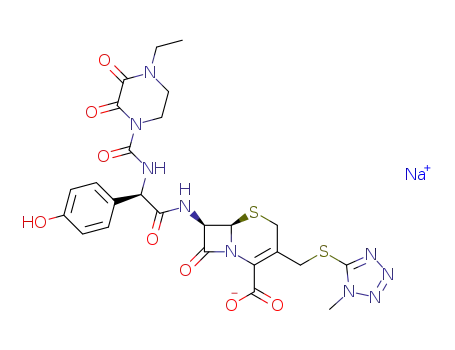
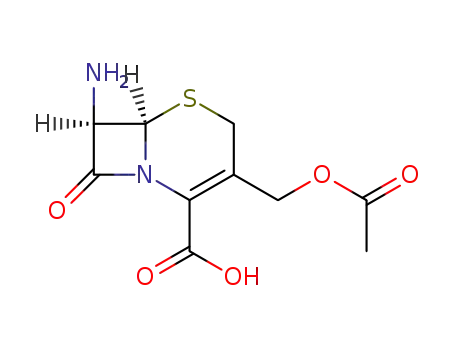
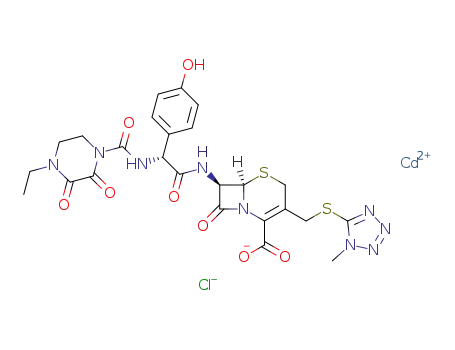
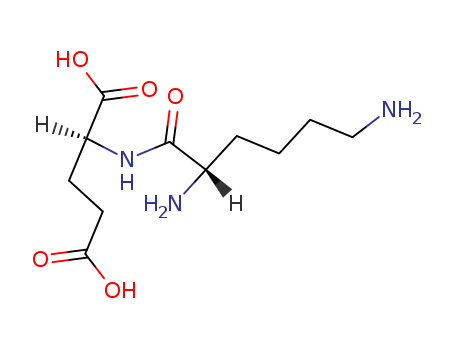
Top Quality Chinese Factory supply 62893-20-3 Cefoperazone sodium
- Molecular Formula:C25H27N9O8S2. Na
- Molecular Weight:667.659
- Appearance/Colour:Faint beige powder
- Melting Point:200-202 °C
- PSA:273.69000
- LogP:-1.85120
Cefoperazone sodium(Cas 62893-20-3) Usage
|
Therapeutic Function
|
Antibiotic |
|
Biological Activity
|
cefoperazone is a new semisynthetic cephalosporin with a broad spectrum of antibacterial activity. cefoperazone shows high activity against gram-positive bacteria and gram-negative bacilli, such as escherichia coli, klebsiella pneumoniae, and proteus species [1]. |
|
Veterinary Drugs and Treatments
|
Cefoperazone is used to treat serious infections, particularly susceptible
Enterobacteriaceae not susceptible to other less expensive
agents or when aminoglycosides are not indicated (due to their potential
toxicity). |
|
in vitro
|
there was only a small spread between the minimum inhibitory concentrations and the minimum bactericidal concentrations of cefoperazone and a significant decrease in activity with an increase in inoculum size. cefoperazone is relatively stable to hydrolysis to β-lactamases produced by gram-negative bacteria. relative rates of hydrolysis of cefoperazone by cephalosporinases were 7.0 to 0.01[1]. in 50 strains of n. gonorrhoeae, the mic50 of cefoperazone was ≤ 0.004-0.06 μg/ml [2]. |
|
in vivo
|
in four patients with cholelithiasis and one patient with carcinoma of the head of the pancreas, all of whom had normal renal functions, cefoperazone was intravenously administrated. in common duct bile, the maximum concentrations of cefoperazone ranged from 373.4 to 3,100 μg/ml while the concentrations ranged from 6.8 to 680 μg/ml in gall bladder bile. cefoperazone concentrations of the gall bladder wall ranged from 16.8 to 48.0 μg/g [3]. |
|
references
|
[1] matsubara n, minami s, muraoka t, et al. in vitro antibacterial activity of cefoperazone (t-1551), a new semisynthetic cephalosporin[j]. antimicrobial agents and chemotherapy, 1979, 16(6): 731-735.[2] baker c n, thornsberry c, jones r n. in vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (ly127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against neisseria gonorrhoeae and haemophilus influenzae, including beta-lactamase-producing strains[j]. antimicrobial agents and chemotherapy, 1980, 17(4): 757-761.[3] nakamura t, hashimoto i, sawada y, et al. cefoperazone concentrations in bile and gall bladder wall after intravenous administration[j]. antimicrobial agents and chemotherapy, 1980, 18(6): 980-982. |
InChI:InChI=1/C25H27N9O8S2.Na/c1-3-32-8-9-33(21(39)20(32)38)24(42)27-15(12-4-6-14(35)7-5-12)18(36)26-16-19(37)34-17(23(40)41)13(10-43-22(16)34)11-44-25-28-29-30-31(25)2;/h4-7,15-16,22,35H,3,8-11H2,1-2H3,(H,26,36)(H,27,42)(H,40,41);/q;+1/p-1/t15-,16-,22-;/m1./s1
62893-20-3 Relevant articles
New adaptation diseases of cefoperazone medicinal preparation for treating endometritis and other gynecological genital tract infections
-
Paragraph 0108; 0012-0013; 0121-0126; 0143; 0144, (2020/08/22)
The invention discloses new adaptation d...
Cefoperazone sodium compound prepared by using fluid mechanics principle and preparation comprising cefoperazone sodium compound
-
Paragraph 0025; 0026; 0037; 0038; 0039-0044; 0054-0057, (2017/05/12)
The invention discloses a cefoperazone s...
Cefoperazone sodium compound and sulbactam sodium compound prepared with strong-field coupling crystallization technology as well as prepared composition
-
Paragraph 0042-0045; 0046; 0047; 0048-0051; 0071-0075, (2017/06/21)
The invention discloses a cefoperazone s...
A NEW CRYSTAL FORM OF CEFOPERAZONE SODIUM
-
Page/Page column 6; 7, (2014/02/15)
The present invention relates to a new c...
62893-20-3 Process route
-
-
62893-19-0,68779-10-2
cefoperazone
Conditions
| Conditions |
Yield |
|
With
sodium hydrogencarbonate;
In
water; acetone;
at 15 - 25 ℃;
for 0.666667h;
pH=6.5 - 6.6;
|
98.1%
|
|
With
sodium isooctanoate; pyrographite;
In
acetone;
at 25 ℃;
pH=6.6;
Reagent/catalyst;
pH-value;
|
71.8%
|
|
With
sodium carbonate;
In
water; ethyl acetate;
at 25 ℃;
pH=6.2;
Temperature;
Solvent;
pH-value;
|
102.61 g
|
|
With
sodium hydrogencarbonate;
In
ethanol; water;
at 30 ℃;
pH=6;
Reagent/catalyst;
Solvent;
Temperature;
pH-value;
|
453g
|
-
-
957-68-6
7-Aminocephalosporanic acid
Conditions
| Conditions |
Yield |
|
Multi-step reaction with 3 steps
1: pyridine; titanium tetrachloride; tetrabutoxytitanium / acetonitrile / 3 h / 5 - 30 °C
2: chloro-trimethyl-silane / N,N-dimethyl-formamide / 4 h / -25 - -20 °C
3: sodium isooctanoate; pyrographite / acetone / 25 °C / pH 6.6
With
pyridine; tetrabutoxytitanium; chloro-trimethyl-silane; sodium isooctanoate; titanium tetrachloride; pyrographite;
In
N,N-dimethyl-formamide; acetone; acetonitrile;
|
|
62893-20-3 Upstream products
62893-20-3 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego