|
Production
|
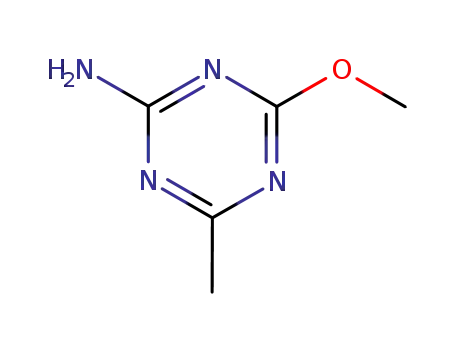
Preparation of 2-mino-4-methyl-6-methoxy symtriazine
The craft process of isourea salt method is as followed: Mix 4.7g cyanamide (90%) with 4g water, add razoxane ethyl ethylimidoote hydrochloride(90%) at 10℃ and stir for 1h. Then use sodium hydroxide to adjust the pH value between 5 to 6 and stir for 1h at 5~10℃. Add 10ml water, stir to layer and obtain N-cyano ethyl ethylimidoote by reduced pressure distillation in oil layer with a yield of 91%. Mix 50g carbamide, 100g dimethyl sulfate and 32g methanol together, heat up it to 55℃slowly, turn off the heat, automatically reach to 60℃, cool it at 68℃ appropriately ,stop cooling at 83℃, continue to heat up to 113℃, then reduce to 105℃automatically and cool to 50℃ to obtain methyl isoureas aimethyl sulfate slat with a yield of 93%. Add 1.4g sodium in 25ml methanol, cool it to -10℃, add 11.2g methyl isoureas aimethyl sulfate slat(90%), drop 5.6g N-cyano ethyl ethylimidoote at a temperature within the range of 0℃ to 14℃, heat it up to 20℃, stir for 20h to obtain 2-mino-4-methyl-6-methoxy symtriazine by post-processing with a yield of 69.3%.
Other synthetic methods can be found in the preparation of metsulfuron-methyl.
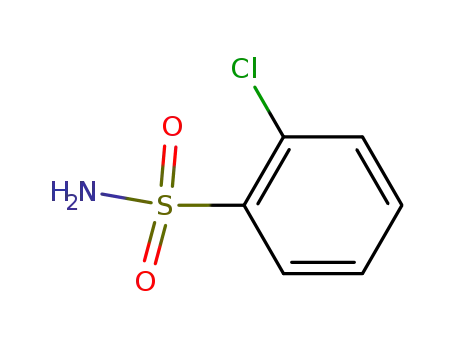
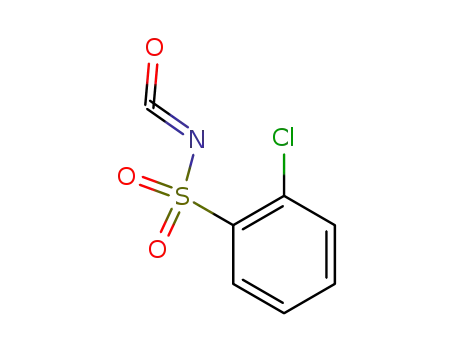
The preparation of N-chlorophenylsulfonyl isocyanate
Mix 230ml concentrated hydrochloric acid, 70ml water and 121g o-chloroaniline together, and cool the mixture to -5℃. Add sodium nitrite solution(40%), keep the reaction temperature below 3℃, add excessive nitrite acid. At the end of the reaction, use a little urea to destroy the excess nitrite, and then remove the solid impurities to complete the diazotization of o-chloroaniline.
Dissolve 198g sodium bisulfite in 350mL water, then divide the solution into 2 parts. One part is put into the reaction flask containing 770ml concentrated hydrochloric acid and 24g anhydrous copper sulfate. Add the other part and diazonium salt solution into the solution above at the same time under the conditions of mixed cooling. Keep the reaction temperature at 0℃, isolate the oil layer at the end of the reaction, wash with water to obtain o-chlorobenzenesulfonyl chloride. The conversion rate of the above two steps is 88.7%. Drop the o-chlorobenzenesulfonyl chloride into a reaction bottle containing 430ml concentrated ammonia water, control the bath temperature at 60℃ and keep the temperature for for 4h. After the filtration, water-dioxane(5:1) mixtures are used to recrystallize, and o-chlorobenzenesulfonamide is obtained after decolorizing with a yield of 63%.
Mix 19.2g o-chlorobenzenesulfonamide, 150ml o-dichlorobenzene and 63.5g oxalyl chloride together, react for 9h to evaporate excess oxalyl chloride. Until the reaction temperature reaches 18℃, the o-dichlorobenzene is evaporated and N-chlorophenylsulfonyl is obtained by reduced pressure distillation with a yield of 57.3%. Phosgene can also be used to replace oxalyl chloride to synthesize cyanate ester in the presence of tertiary amine.
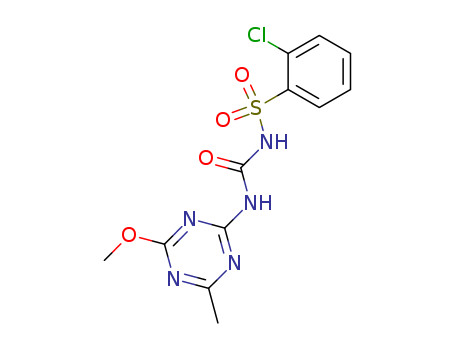
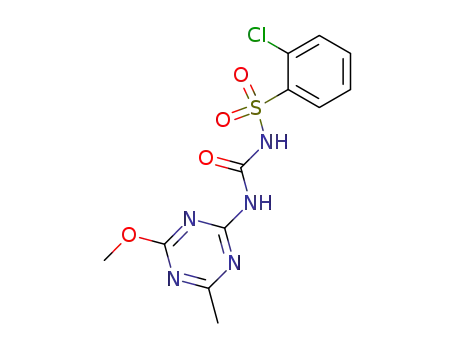
Synthesis of chlorsulfuron
Mix 0.02mol 2-mino-4-methyl-6-methoxy symtriazine and 40ml anhydrous acetonitrile together, drop 0.02mol N-chlorophenylsulfonyl isocyanate acetonitrile solution into the mixture and then continue to stir at the room temperature for 24h. Sniff out white powders, wash with acetonitrile, dry to obtain chlorsulfuron products with a yield of 70%. Chlorsulfonide can also be prepared by the addition reaction of o-chlorobenzenesulfonamide with 4-methyl-6-methoxy symtriazine-2-isocyanate prepared by the reaction of 2-mino-4-methyl-6-methoxy symtriazine and phosgene. |
|
Air & Water Reactions
|
Insoluble in water. Reacts slowly with water. The reaction is promoted by acid such that the pH is less than 5.0 (1/2 life of 24-48 hrs.). Reaction is also promoted by polar organic solvents such as methanol and acetone. |
|
Trade name
|
DPX 4189?; FINESSE?; GLEAN?;
GLEAN 20DF?; LANDMARK? MP; LASHER?;
RIVERDALE CORSAIR?; TELAR? DF |
|
Potential Exposure
|
A selective systemic sulfonylurea herbicide used to control most broadleaf weeds and some annual grasses in wheat, barley, oats, duram, rye, triticale, and flax. Applied to noncrop sites such as rights-of-way, fence rows, and roadsides. |
|
Environmental Fate
|
Soil. Degrades in soil via hydrolysis followed by microbial degradation forming low
molecular weight, inactive compounds. The estimated half-life was reported to range from
4 to 6 weeks (Hartley and Kidd, 1987; Cremlyn, 1991). Microorganisms capable of
degrading chlorsulfuron are Aspergillis niger, Streptomyces griseolus and Penicillium sp.
(Humburg et al., 1989). One transformation product reported in field soils is 2-chlorobenzenesulfonamide (Smith, 1988)The reported dissipation rate of chlorsulfuron in surface soil is 0.024/day (Walker and
Brown, 1983). The persistence of chlorsulfuron decreased when soil temperature and
moisture were increased (Walker and Brown, 1983; Thirunarayanan et al., 1985)Plant. Chlorsulfuron is metabolized by plants to hydroxylated, nonphytotoxic compounds including 2-chloro-N-(((4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino)carbonyl)benzenesulfonamide (Duke et al., 1991). Devine and Born (1985) and Peterson andPhotolytic. The reported photolysis half-lives of chlorsulfuron in distilled water, methanol and natural creek water at λ >290 nm were 18, 92 and 18 hours, respectively. In all
cases, 2-chlorobenzene sulfonamide, 2-methoxy-4-methyl-6-amino-1,3,5-triazine and
trace amounts of the tentatively identified compound nitroso-2-chlorophenylsulfone
formed as photoproducts (Herrmann et al., 1985). |
|
Metabolic pathway
|
Chlorsulfuron is metabolized in wheat and in tolerant
broadleaves via different pathways where
hydroxylation occurs on the methyl group of the
triazine ring and at the phenyl ring of the chlorsulfuron
in respective plants. With chemical degradation of
chlorsulfuron on dry minerals (Syst.), two pathways of
degradation are observed, one of which is direct |
|
Toxicity evaluation
|
Chlorsulfuron has a moderate to short-lived fate in the environment.
It does not bioaccumulate and is not volatile. In the
environment, chlorsulfuron degrades via a combination of
biotic and abiotic processes. Chlorsulfuron degrades in acidic
solutions and soil by cleavage of the sulfonylurea bridge, Odemethylation,
and hydroxylation. Chlorsulfuron is metabolized
by soil microbes to numerous minor degradation products, is mineralized to CO2, and sequestered as nonextractable
residues. Photodegradation is not a significant
pathway of dissipation for chlorsulfuron in the environment.
Hydrolytic processes are not expected to be a major contributing
factor in the environmental degradation of chlorsulfuron,
and would only be significant at acidic pH. |
|
Incompatibilities
|
Slowly hydrolyzes in water, releasing ammonia and forming acetate salts. May bencompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. |
|
Waste Disposal
|
It is the responsibility of chemical waste generators to determine the toxicity and physical properties and of a discarded chemical and to properly identify its classification and certification as a hazardous waste and to determine the disposal method. United States Environmental Protection Agency guidelines for the classification determination are listed in 40 CFR Parts 261.3. In addition, waste generators must consult and follow all regional, national, state, and local hazardous waste laws to ensure complete and accurate classification and disposal methods. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations |
|
General Description
|
Colorless crystals. Non corrosive. Insoluble in water. Used as an herbicide. |
|
Agricultural Uses
|
Herbicide: A selective systemic herbicide used to control most
broadleaf weeds and some annual grasses in wheat, barley,
oats, duram, rye, triticale and flax. Applied to non-crop
sites such as rights-of-way, fence rows and roadsides. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego