|
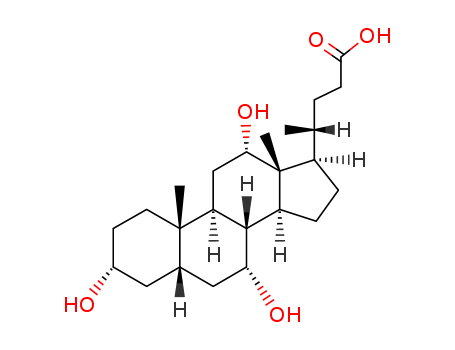
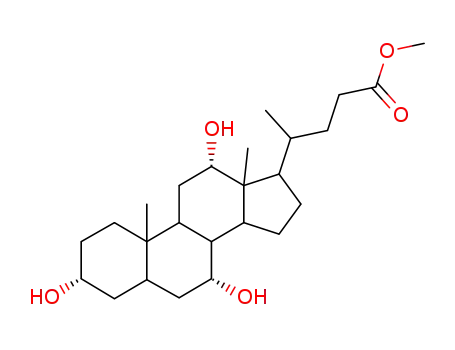
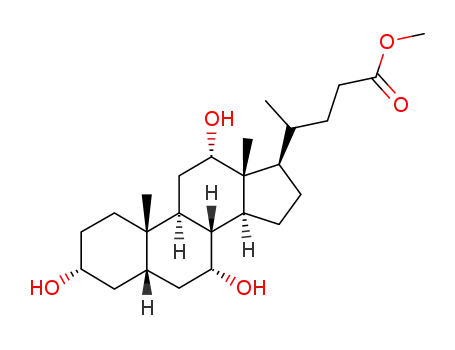
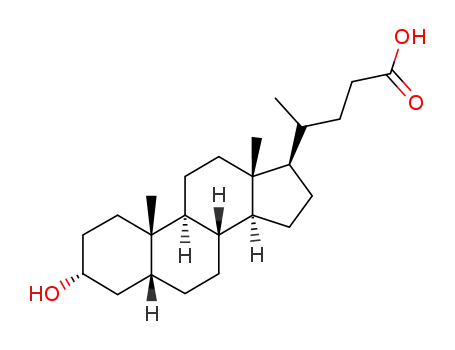
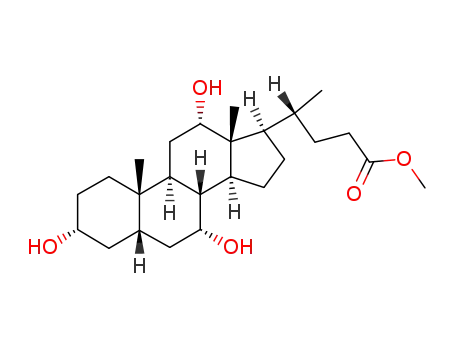
Chemical properties
|
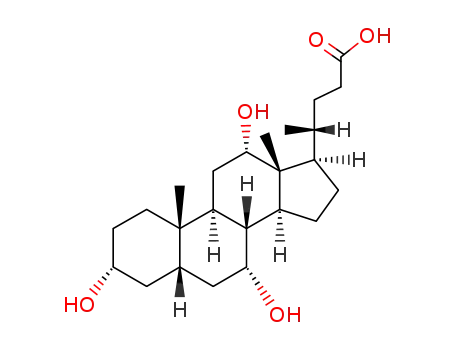
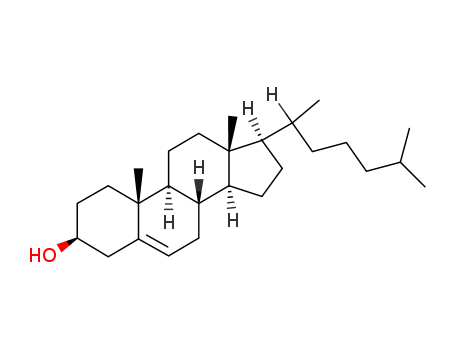
This agent exist in the bile cattle, sheep, pig. It is a colorless sheet or white crystalline powder. Some have a bitter to sweet taste. Its melting point is 198℃, specific rotation is (c = 0.6, ethanol) +37°. 1g cholic acid dissolved in about 300ml ethanol or acetone, 7ml glacial acetic acid. A small amount of cholic acid is soluble in water. The monohydrate was a white flake crystal. In 1927, H.Wieland (Germany) research accomplished bile acid composition, and won the Nobel Prize in chemistry. |
|
Identification test
|
Solubility: hardly insoluble in water; soluble in ethanol. According to OT-42 method.
The melting range : 197~202 ℃. As determined by conventional methods.
Add 50% acetic acid solution to prepare 0.02% of the sample solution; take 1ml, 1% furfural solution, 6ml of water and concentrated sulfuric acid 5ml. This mixture should be converted into rosiness in 5min and then turn purple.
Take about 10mg sample, add 2 drops of benzaldehyde and 3: 1 sulfuric acid 3 drops, heating at 50 ℃ for 5min. Plus glacial acetic acid about 10ml, then it should be brown. |
|
Production methods
|
(1) Extracting from livestock (pigs, cattle, sheep, rabbits) the bile.
1.Ethanol crystallization method
Preparation of crude cholic acid of cattle, sheep: take bovine or sheep bile; add 100 g/L sodium hydroxide; heat to boil for 12-18h to get saponification solution. Cooling. Adding acid to pH 1, precipitating cholic acid, removing bile acid, undergoing boiling and rinsing, drying at 75℃, milling can obtain bovine cholic acid.
sodium cholate[NaOH] → [100 ℃, 12-18h] saponified solution[H2SO4] → [pH1, 75 ℃] crude cholic acid of cattle, sheep.
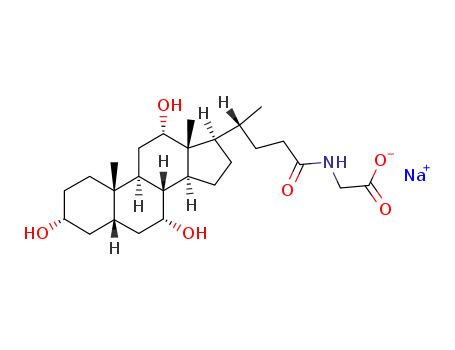
Preparation of sodium cholate: add 0.5-1 times of crude cholic acid to 95% ethanol, and dissolve the solids by heating reflux method; cool. Broke the crystallization; filter; add 95% washing ethanol to make the filtrate colorless. Crystallize by adding 4 times the amount of ethanol; add 100-150g/L of activated carbon; dissolve the solids by heating reflux method; filter the liquid when it is hot. The filtrate concentrated to the original volume of 1/4. Through cooling, crystallization, filtration, adding ethanol to wash the crystallization can obtain the sodium cholate products.
Crude cholic acid cattle, sheep [ethanol, activated carbon] → refined liquid [90 ℃ below] → sodium cholate products
2.? Ethyl acetate separation method
Preparation of crude hyocholic acid: Add 3-3.5 times of saturated lime supernatant into fresh pig bile under stirring, then continue to stir it for 5-10min. heating to boiling for 2min, cooling, through filtration, adding hydrochloric acid to PH3.5 to get precipitation, standing for more than 12h can obtain crude acid. Removing, washing, adding 1.5 times the sodium hydroxide, plus 9 times the water, heating and boiling 12-18h, cooling, standing overnight obtain paste. Add water and sulfuric acid to pH 1 to precipitate pig cholic acid. Removing, crushing, rinsing to no acidity, through filtration gains crude pig cholic acid.
[pig bile] [saturated limewater] →[100℃, pH 11-12] Basic filtrate [HCl] → [pH3.5] Crude cholic acid [water, NaOH] → paste [H2SO4] → [pH1] crude pig cholic acid
Preparation of pig cholic acid products: add 4 times the amount of ethyl acetate to the crude pig cholic acid. Add 150-200g/L activated carbon; heat and flux for 0.5h; cool; filter; add 1.5-2.5 times ethyl acetate to filter cake; combine the filtrates twice. Adding 200g/L anhydrous sodium sulfate, standing overnight, concentrated to the original volume of 1/3, releasing, cooling crystallization, through filtration, washing with ethyl acetate crystallization, drying can obtain pig cholic acid products.
Crude pig cholic acid [ethyl acetate, activated carbon] → filtrate [anhydrous sodium sulfate] → filtrate [concentration] → pig cholic acid products. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego