|
Antiviral drugs
|
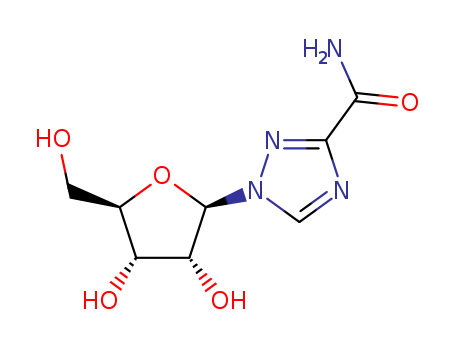
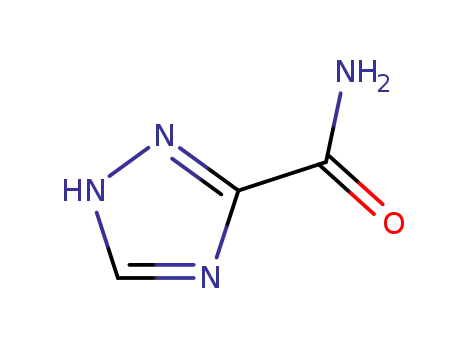
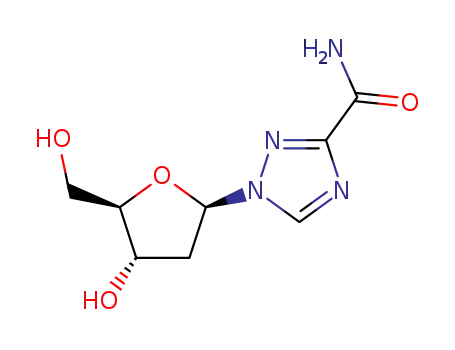
Ribavirin is a novel non-selective nucleoside class broad-spectrum antiviral drug, belonging to the inosine monophosphate (IMP) dehydrogenase inhibitor. It can participate in the guanine participate in the human body metabolism, interfere with the biosynthesis of guanine, prevent the replication of the virus and has inhibitory effect on a variety of DNA and RNA viruses. Ribavirin has a stronger antiviral effect than amantadine and vidarabine, etc with definite efficacy and small drug side effects. Rare adverse reactions include conjunctivitis and hypotension with the former exhibiting as intraocular foreign body sensation, photophobia, itching, swelling and hematoma and the latter manifested as blurred vision, dizziness, and fatigue. It is used for clinical treatment of viral upper respiratory tract infection, respiratory syncytial virus-induced viral pneumonia and bronchitis, influenza, para-influenza, epidemic encephalris, mumps, chickenpox, shingles, herpes zoster, autumn diarrhea, children early-stage adenovirus pneumonia, acute Lassa fever, rubella, viral pneumonia, genital herpes, herpes simplex virus keratitis, psoriasis, etc with special efficacy. It also have certain efficacy on treating epidemic hemorrhagic fever, hepatitis B, and hepatitis A. Applying this drug in early stage of epidemic hemorrhagic fever can shorten the period, reducing the damage of kidney and vascular and symptoms of poisoning. |
|
Pharmacological effects
|
1, Ribavirin can be subject to phosphorylation in red blood cells to generate ribavirin monophosphate, diphosphate and triphosphate, wherein the ribavirin monophosphate is the strong inhibitor of the inosine monophosphate dehydrogenase which can inhibit cellular guanylate synthesis, decrease the tri-phosphorylation of the intracellular guanylate triphosphate, and blocking the synthesis of viral nucleic acid.
2, ribavirin triphosphate can inhibit the influenza virus RNA polymerase and interfere with viral replication.
3, ribavirin triphosphate can inhibit the synthesis of viral mRNA 5 'end and transcriptase, thereby inhibiting the DNA and RNA synthesis. It has inhibitory effect on various kinds of viruses (including DNA viruses and RNA viruses). It also has prevention and treatment effect on the influenza, adenovirus pneumonia, hepatitis, herpes, and measles and is also effective on treating the epidemic hemorrhagic fever.
4, it is mainly excreted through urine in the prototype with also a small amount of de-sugarized ribavirin metabolites with a small amount discharged from the feces.
The above information is edited by the lookchem of Dai Xiongfeng. |
|
Production method
|
Take nucleotide or nucleoside as the starting material
First hydrolyze the guanosine and guanylate upon the action of glacial acetic acid and acetic anhydride to generate ribose-1-phosphate, which is then subject to the catalysis of double-para-nitro phenol to have reaction with triazide amide to generate condensate with aminolysis to obtain the product.
Guanosine (or guanylate) [acetic anhydride, glacial acetic acid]→[hydrolysis] ribose-1-phosphate [bis-(p-nitrophenol)-phosphate]→[triazide carboxamide] condensates [aminolysis]→Ribavirin
Enzymatic synthesis
First hydrolyze the guanosine and guanosine acid under the action of pyrimidine nucleoside phosphorylase to form ribose-1-phosphate which then, under the action of purine nucleoside phosphorylase, has reaction with triazide amide to directly generate triazole nucleosides.
Guanosine (or guanylate) [pyrimidine nucleoside phosphorylase] → ribose-1 nucleic acid [purine nucleotide cyclase] → Ribavirin. |
|
Indications
|
Ribavirin is a synthetic guanosine analogue that possesses
broad antiviral inhibitory activity against many
viruses, including influenza A and B, parainfluenza,RSV,
HCV, HIV-1, and various herpesviruses, arenaviruses,
and paramyxoviruses. Its exact mechanism of action has
not been fully elucidated; however, it appears to inhibit
the synthesis of viral mRNA through an effect on nucleotide
pools. Following absorption, host cell enzymes
convert ribavirin to its monophosphate, diphosphate,
and triphosphate forms. Ribavirin monophosphate inhibits the guanosine triphosphate (GTP) synthesis
pathway and subsequently inhibits many GTP-dependent
processes. Ribavirin triphosphate inhibits the 5 capping
of viral mRNA with GTP and specifically inhibits
influenza virus RNA polymerase. Ribavirin may also
act by increasing the mutation rate of RNA viruses, leading
to the production of nonviable progeny virions.
Ribavirin resistance has not been documented in clinical
isolates. |
|
Acquired resistance
|
Development of resistant virus strains has not been
demonstrated. |
|
Air & Water Reactions
|
Water soluble. |
|
Reactivity Profile
|
Ribavirin may be sensitive to prolonged exposure to light. |
|
Hazard
|
Mildly toxic by ingestion. An experimental
teratogen. |
|
Fire Hazard
|
Flash point data for Ribavirin are not available; however, Ribavirin is probably combustible. |
|
Pharmaceutical Applications
|
A synthetic nucleoside. It is neither a classic pyrimidine nor a
purine, but stereochemical studies indicate that it is a guanosine
analog. It is usually formulated for administration by inhalation,
but oral and intravenous preparations are also used. |
|
Biochem/physiol Actions
|
Antiviral agent used against a wide variety of human viral infections, in particular, chronic hepatitis?C, HIV, and adenovirus. Its metabolite, ribavirin 5′-phosphate, is an inhibitor of inosine monophosphate (IMP) dehydrogenase, but many other mechanisms of action are also supported with experimental evidence. |
|
Mechanism of action
|
Ribavirin, a guanosine analogue, has broad-spectrum antiviral activity against both DNA and RNA viruses. It is phosphorylated by adenosine kinase to the triphosphate, resulting in the inhibition of viral
specific RNA polymerase, disrupting messenger RNA and nucleic acid synthesis. |
|
Pharmacology
|
Oral and intravenous ribavirin are associated with
additional adverse effects.When given via these routes,
ribavirin can produce hemolytic anemia that is reversible
following dosage reduction or cessation of therapy.
When given in combination with interferon- , ribavirin
increases the incidence of many of its side effects,
such as fatigue, nausea, insomnia, depression, and anemia,
and may cause fatal or nonfatal pancreatitis.
Ribavirin is mutagenic, teratogenic, and embryotoxic in
animals at doses below the therapeutic level in humans.
It is contraindicated in pregnant women and in the male
partners of pregnant women. Women of childbearing
potential and male partners of these women must use
two effective forms of contraception during ribavirin
treatment and for 6 months post therapy. Pregnant
women should not directly care for patients receiving
ribavirin. |
|
Pharmacokinetics
|
Oral absorption: 36–46%
Cmax 3 mg/kg oral: 4.1–8.2 μmol/L after 1–1.5 h
600 mg intravenous: 43.6 μmol/L end infusion
Plasma half-life: c. 24 h
Volume of distribution: 647 L
Plasma protein binding: <10%
Absorption
It is rapidly absorbed after oral administration. Mean peak concentrations after 1 week of oral doses of 200, 400 and 800 mg every 8 h were 5.0, 11.1 and 20.9 μmol/L, respectively. Trough levels 9–12 h after the end of 2 weeks’ therapy were 5.1, 13.2 and 18.4 μmol/L, respectively, indicating continued accumulation of the drug. Drug was still detectable 4 weeks later. Mean peak plasma concentrations after intravenous doses of 600, 1200 and 2400 mg were 43.6, 72.3 and 160.8 μmol/L, respectively; at 8 h the mean plasma concentrations were 2.1, 5.6 and 10.2 μmol/L. Aerosolized doses (6 g in 300 mL distilled water) are generally administered at a rate of 12–15 mL/h using a Collison jet nebulizer, the estimated dosage being 1.8 mg/kg per h for infants and 0.9 mg/kg per h for adults. When administered by small particle aerosol for 2.5–8 h, plasma concentrations ranged from 0.44 to 8.7 μmol/L.
Metabolism and excretion
It is rapidly degraded by deribosylation or amide hydrolysis, and together with its metabolites is slowly eliminated by the kidney. About 50% of the drug or its metabolites appear in the urine within 72 h and 15% is excreted in the stools. The remainder seems to be retained in body tissues, principally in red blood cells, which concentrate the drug or metabolites to a peak at 4 days, with a half-life of around 40 days. After intravenous administration 19.4% of the dose was eliminated during the first 24 h (compared with 7.3% after an oral dose), the difference reflecting the bioavailability. |
|
Side effects
|
Most adverse effects associated with aerosol ribavirin
are local. Pulmonary function may decline if aerosol ribavirin
is used in adults with chronic obstructive lung disease
or asthma. Deterioration of pulmonary and cardiovascular
function has also been seen in severely ill
infants given this preparation. Rash, conjunctivitis, and
rare cases of anemia have been reported. Health care
workers exposed to aerosol ribavirin during its administration
have reported adverse effects including headache,
conjunctivitis, rash, and rarely, bronchospasm. |
|
Synthesis
|
Ribavirin, 1-β-D-ribofuranosyl-1H-1,2,4-triazol-3-carboxamide (36.1.28), is
synthesized by reacting methyl ester of 1,2,4-triazol-3-carboxylic acid with O-1,2,3,
5-tetraacetyl-β-D-ribofuranose to make methyl ester of 1-O-2,3,5-tetraacetyl-β-D-ribofura�nosyl-1,2,4-triazol-3-carboxylic acid (36.1.27), which is treated with an ammonia solution
of methanol to simultaneously dezacylate the carbohydrate part and amidation of the car�boxyl part of the product to give ribavirin. |
|
Drug interactions
|
Potentially hazardous interactions with other drugs
Antivirals: effects possibly reduced by abacavir;
increased risk of toxicity with stavudine; increased
side effects with didanosine - avoid; increased risk of
anaemia with zidovudine - avoid.
Azathioprine: possibly enhances myelosuppressive
effects of azathioprine. |
|
Metabolism
|
Ribavirin is metabolised by reversible phosphorylation
and a degradative pathway involving deribosylation and
amide hydrolysis to produce an active triazole carboxyacid
metabolite.
Ribavirin is mainly excreted in the urine as unchanged
drug and metabolites. |
|
Definition
|
ChEBI: A 1-ribosyltriazole that is the 1-ribofuranosyl derivative of 1,2,4-triazole-3-carboxamide. An inhibitor of HCV polymerase. |
|
Brand name
|
Copegus (Roche); Rebetol (Schering); Virazole (Valeant). |
|
General Description
|
White powder. Exists in two polymorphic forms. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego